Occipital lobe activities: Neuroanatomy, Occipital Lobe – StatPearls
Neuroanatomy, Occipital Lobe – StatPearls
Amna Rehman; Yasir Al Khalili.
Author Information
Last Update: July 31, 2021.
Introduction
The occipital lobe is the smallest of the four lobes of the cerebral hemisphere. It is present posterior to the parietal and temporal lobes. Thus, it forms the caudal part of the brain. Relative to the skull, the lobe lies underneath the occipital bone. It rests on the tentorium cerebelli, which separates it from the cerebellum. The paired occipital lobes are separated from each other by a cerebral fissure. The posteriormost part of the occipital lobe is known as the occipital pole.
The occipital lobe is primarily responsible for visual processing. It contains the primary and association visual cortex.
Structure and Function
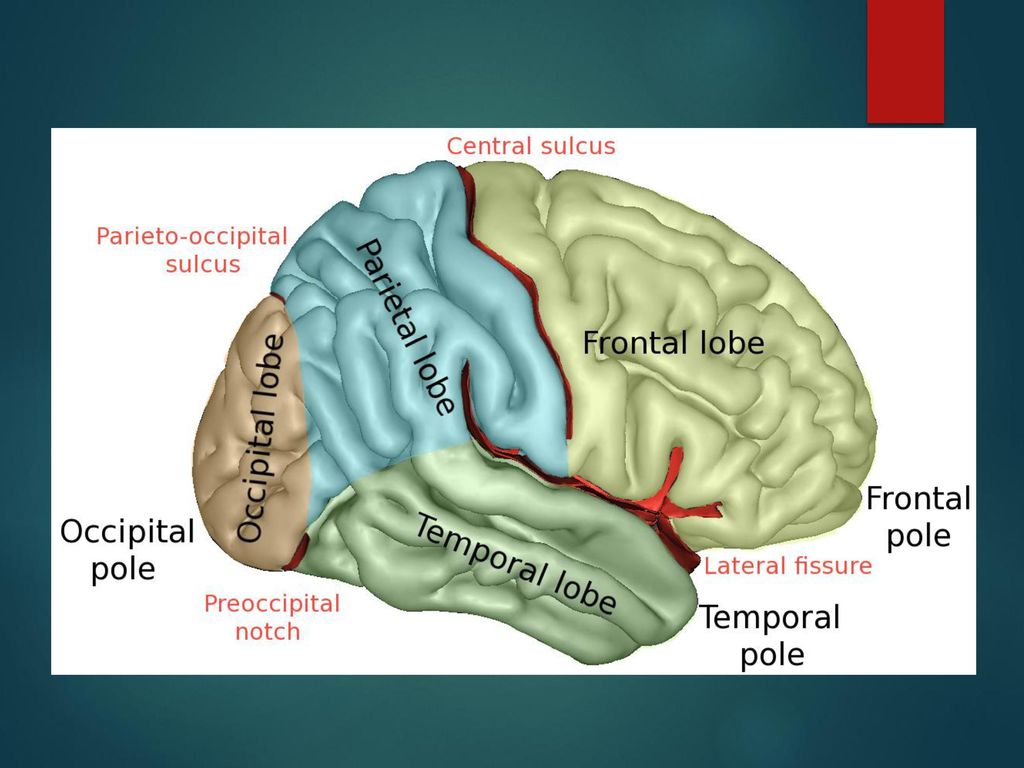
The demarcation of the occipital lobe from the parietal and temporal lobe on the medial surface by the parieto-occipital sulcus and on the lateral surface by an imaginary line that extends from parieto-occipital sulcus to the preoccipital notch. [1]
The cerebral surface of the occipital lobe irregularly molds into eminences called gyri and separated by depressions called sulci. The lateral surface of the occipital lobe consists of three characteristic occipital sulci: the intra-occipital sulcus, the transverse occipital sulcus, and the lateral occipital sulcus. The intra-occipital sulcus is an extension of the intraparietal sulcus of the parietal lobe. The transverse occipital sulcus crosses the superolateral surface of the brain transversely and lies posterior to the parieto-occipital sulcus. The lateral occipital sulcus is a horizontal sulcus that divides the lateral occipital surface into gyri. The occipital lobe commonly subdivides into superior and inferior gyri by the lateral occipital sulcus. Occasionally it divides into three occipital gyri; superior, middle, and inferior, by the lateral occipital sulcus and extension of the transverse occipital sulcus. The superior and inferior gyri converge to form the occipital pole.
The medial surface of the occipital lobe has a characteristic calcarine sulcus (calcarine fissure). It extends from the parieto-occipital sulcus to the occipital pole. The upper and lower banks of calcarine sulcus house the primary visual cortex. Each primary visual cortex receives visual information from the contralateral half of the brain. The calcarine sulcus divides the medial surface into cuneus (superior gyrus) and lingual (inferior gyrus). The fusiform gyrus extends from the temporal lobe and lies below the lingual gyrus.
The occipital lobe is the visual processing area of the brain. It is associated with visuospatial processing, distance and depth perception, color determination, object and face recognition, and memory formation. The primary visual cortex, also known as V1 or Brodmann area 17, surrounds the calcarine sulcus on the occipital lobe’s medial aspect. It receives the visual information from the retina via the thalamus. The secondary visual cortex, also known as V2, V3, V4, V5, or Brodmann areas 18 and 19, surrounds the primary cortex and receives information from it.
The primary visual cortex transmits information through two pathways: the dorsal and ventral stream. The dorsal stream is associated with object location and carries visual information to the parietal lobe. The ventral stream has associations with object recognition and transmits visual information to the temporal lobe.
Embryology
The occipital lobe of the cerebral cortex derives from the telencephalon. Thus, its embryologic development is associated with the development of telencephalon. At two weeks of conception, the embryo is a two-layered structure. During the third week, gastrulation divides the embryo into three layers – the endoderm, mesoderm, and ectoderm. The ectodermal stem cells give rise to the brain and central nervous system.
The neuroectodermal cells become arranged in the midline forming the neural plate. At the end of the third developmental week, the neural plate ends approximate and fuse to form the neural tube. By week 4, the neural tube expands to form three characteristic cavities called the brain vesicles.
The telencephalon undergoes growth and infoldings to form two cavities. The cavities give rise to cerebral hemispheres and the lateral ventricles. At five months, the hemispheres have expanded to occupy most of the brain cavity. At eight months, the gyri and sulci become prominent.[4]
Blood Supply and Lymphatics
The occipital lobe receives vascular supply from the cortical branches of the posterior cerebral artery (PCA). The parieto-occipital
artery originates from the distal segment of PCA in the calcarine sulcus. It supplies the parieto-occipital sulcus and some areas of cuneus.
As the name suggests, it supplies the lingual gyrus. The posterior-temporal artery supplies the lingual gyrus and caudal portion of the fusiform gyrus. The common temporal artery is also known as the lateral occipital artery or temporooccipital artery. It supplies the fusiform gyrus and sometimes lingual gyrus. Various neurologic deficits can occur as a result of occlusion of these branches, because of the variability of the regions supplied by the tributaries of the PCA.[5]
Surgical Considerations
Surgery of the occipital lobe is important for lesions such as gliomas and metastases. Neurosurgeons can approach these lesions through a trans-sulcal approach, or use sulci as an anatomical guide to perform en bloc resection. Sometimes surgeons access the occipital lobe to approach deeper structures of the brain.
Surgery has also proven successful in patients of medically-resistant occipital lobe epilepsy (OLE). However, it is challenging to identify the entire epileptogenic zone on magnetic resonance imaging (MRI) or ictal electroencephalography (EEG). Additionally, resection of the lesion is often limited in an attempt to preserve the visual field, often leading to worse outcomes. Postoperative visual deficits are common with OLE surgery.[6]
Clinical Significance
Injury to the occipital lobe can occur due to vascular insults, neoplastic lesions, trauma, infections, and seizures.
Unilateral occipital lobe lesion causes contralateral homonymous hemianopia. It is a visual field defect on the same side of both eyes contralateral to the site of the lesion — lesions of the occipital lobe due to the posterior cerebral artery infarct cause homonymous hemianopia with macular sparing. Macular sparing is due to the dual blood supply of the occipital pole by middle and posterior cerebral arteries. Lesions of the posterior occipital lobe may cause homonymous hemianopia with sparing of crescent-shaped
temporal vision. The posterior lobe lesions spare the anterior striate cortex, which controls temporal vision. Bilateral occipital lesions cause bilateral complete hemianopia, also known as cortical blindness.
Anton syndrome is sometimes present in patients with cortical blindness. It occurs in cases of insult to the occipital lobe. The patient persistently denies loss of vision and is unaware of the visual deficit, despite evidence of cortical blindness.
Another rare syndrome associated with occipital lobe injury is Riddoch syndrome. The person is only able to see moving objects in the blind field, while non-moving objects are invisible. The person has motion perception while unable to perceive shape or color.[9]
Occipital lobe epilepsy is relatively uncommon but often presents with specific neurological findings. Seizures originating in the occipital lobe are associated with visual hallucinations, blurring or loss of vision, and rapid eye blinking or fluttering of eyelids. The seizures usually occur after a bright visual image or flicker stimulus.[10]
Occipital lobe lesions can cause visual hallucinations, color agnosia, or agraphia.
Review Questions
-
Access free multiple choice questions on this topic.
-
Comment on this article.
Figure
Principal fissures and lobes of the cerebrum viewed laterally, Frontal Lobe, Parietal Lobe, Temporal Lobe, Occipital Lobe.
References
- 1.
-
Flores LP. Occipital lobe morphological anatomy: anatomical and surgical aspects. Arq Neuropsiquiatr. 2002 Sep;60(3-A):566-71. [PubMed: 12244393]
- 2.
-
Alves RV, Ribas GC, Párraga RG, de Oliveira E. The occipital lobe convexity sulci and gyri. J Neurosurg. 2012 May;116(5):1014-23. [PubMed: 22339163]
- 3.
-
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010 Dec;20(4):327-48. [PMC free article: PMC2989000] [PubMed: 21042938]
- 4.
-
Patel A, Biso GMNR, Fowler JB. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 31, 2021. Neuroanatomy, Temporal Lobe. [PubMed: 30137797]
- 5.
-
Marinković SV, Milisavljević MM, Lolić-Draganić V, Kovacević MS. Distribution of the occipital branches of the posterior cerebral artery. Correlation with occipital lobe infarcts. Stroke. 1987 Jul-Aug;18(4):728-32.
[PubMed: 3603599]
- 6.
-
Harward SC, Chen WC, Rolston JD, Haglund MM, Englot DJ. Seizure Outcomes in Occipital Lobe and Posterior Quadrant Epilepsy Surgery: A Systematic Review and Meta-Analysis. Neurosurgery. 2018 Mar 01;82(3):350-358. [PMC free article: PMC5640459] [PubMed: 28419330]
- 7.
-
Maddula M, Lutton S, Keegan B. Anton’s syndrome due to cerebrovascular disease: a case report. J Med Case Rep. 2009 Sep 09;3:9028. [PMC free article: PMC2827161] [PubMed: 20226004]
- 8.
-
M Das J, Naqvi IA. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Apr 10, 2021. Anton Syndrome. [PubMed: 30844182]
- 9.
-
Zeki S, Ffytche DH. The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain. 1998 Jan;121 ( Pt 1):25-45. [PubMed: 9549486]
- 10.
-
Emmady PD, M Das J. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 6, 2021. Benign Occipital Seizure.
[PubMed: 32491402]
Occipital Lobe: Function, Location, and Structure
The Occipital Lobe helps with visual processing and mapping. It is located under the parietal lobe and above the temporal lobe near the back of the brain.
The occipital lobe is the seat of most of the brain’s visual cortex, allowing you not only to see and process stimuli from the external world, but also to assign meaning to and remember visual perceptions. Located just under the parietal lobe and above the temporal lobe, the occipital lobe is the brain’s smallest lobe, but its functions are indispensable.
Where is the Occipital Lobe Located?
Understanding the occipital lobe requires a basic understanding of brain anatomy. The cerebral cortex of the brain—a part of the brain shared by all vertebrates—is the newest part of the brain, evolutionarily speaking. All mammalian brains have four distinct lobes, but the brain itself—as well as the lobes it contains—is divided into right and left hemispheres.
Like other lobes of the brain, the occipital lobe does not have clear internal boundaries separating it from the rest of the brain. Instead, neuroscientists use the skull’s bones as their guide, so the occipital lobe rests underneath the occipital bone.
The occipital lobe is the rearmost lobe of the brain, located in the forebrain. It rests upon the tentorium cerebelli, a thick membrane of tissue the separates the cerebrum from the evolutionarily older cerebellum.
What Does the Occipital Lobe Do?
Studying the brain is a difficult task, particularly since some areas compensate for others when the brain suffers damage. The brain’s sensitive, dense, and complex nature means that researchers are constantly uncovering new structures within the brain, and new functions for each brain lobe.
Researchers once thought that the occipital lobe only controlled visual functions. But in recent years, they discovered that some portions of this lobe receive inputs from other brain regions. Specifically, a brain region called the dorsomedial stream receives input both from regions of the brain related to vision, and to areas that are not linked to visual processing. This suggests either that the occipital lobe may perform additional functions, or that researchers have not identified all regions of the brain associated with visual processing.
Although we know that the occipital lobe is dedicated to vision, this process is highly complex, and includes a number of separate functions. Those include:
- Mapping the visual world, which helps with both spatial reasoning and visual memory. Most vision involves some type of memory, since scanning the visual field requires you to recall that which you saw just a second ago.
- Determining color properties of the items in the visual field.
- Assessing distance, size, and depth.
- Identifying visual stimuli, particularly familiar faces and objects.
- Transmitting visual information to other brain regions so that those brain lobes can encode memories, assign meaning, craft appropriate motor and linguistic responses, and continually respond to information from the surrounding world.
- Receiving raw visual data from perceptual sensors in the eyes’ retina.
What Are Some Important Structures in the Occipital Lobe?
Like all other lobes of the brain, the occipital lobe contains a number of structures and neuronal tracts that work together to enable vision. Those include:
- Brodmann area 17: Known as V1, this region is located in the occipital lobe’s calcarine sulcus, and serves as the brain’s primary visual cortex. It aids the brain to determine location, spatial information, and color data.
- The ventral stream: Known sometimes as V2, this is a secondary visual cortex that helps the brain assign meaning to what it is seeing. Without V2, you would still be able to see, but would have no conscious awareness of or understanding of the sights your eyes took in.
- The dorsomedial stream: Neuroscientists don’t yet have a strong understanding of this brain region, which connects to both V1 and V2, as well as other brain regions.
- The lateral geniculate bodies: These structures take in optic information from retinal sensors in each eye, sending raw information to each visual cortex.
- Lingula: this area receives information from the contralateral inferior retina to gather information about the field of vision.
Brain imaging studies have revealed that neurons on the back of the gray matter of the occipital lobe create an ongoing visual map of data taken in by the retinas.
How Does the Occipital Lobe Interact With Other Areas of the Body?
No part of the brain is a standalone organ that can function without information from other parts of the body. The occipital lobe is no exception. Although its primary role is to control vision, damage to other brain regions and body parts can inhibit vision. Moreover, some evidence suggests that, when the occipital lobe is damaged, nearby brain regions may be able to compensate for some of its functions. The occipital lobe is heavily dependent on:
- The eyes, particularly the retinas, which take in and process visual information to then be further processed by the occipital lobe.
- The frontal lobe, which contains the brain’s motor cortex. Without motor skills, the eyes cannot move or take in information from surrounding regions.
- The temporal lobe, which helps assign meaning to visual information, in addition to encoding it into memories.
What Happens if the Occipital Lobe is Damaged?
The most obvious effect of damage to the occipital lobe is blindness, but occipital lobe damage can have other surprising effects:
- Epilepsy: Some seizures occur in the occipital lobe, and occipital lobe damage increases vulnerability to seizures.
- Difficulties with movement: Even if you are still able to move, changes in depth perception and vision can lead to inappropriate movements and difficulty navigating the visual field.
- Difficulties perceiving colors, shape, dimension, and size.
- Difficulty recognizing familiar objects or faces.
- Hallucinations
- Inability to recognize or read written words.
- Inability to detect that an object is moving.
- Difficulty reading or writing; for example, the words may appear to move on the page.
- Difficulty locating objects within the environment, even when you are able to see those objects.
- Difficulties with fine and gross motor skills, as well as balance.
Sources
- Occipital Lobe: Facts, Position In Brain, Summary & Function. Brain Made Simple. Published September 26, 2019. Accessed May 11, 2020. Learn More.
- Rehman A. Neuroanatomy, Occipital Lobe. StatPearls [Internet]. Published July 6, 2019. Accessed May 11, 2020. Learn More.
- Occipital lobe. Healthline. Accessed May 11, 2020.
- Occipital Lobes. Centre For Neuro Skills. Accessed May 11, 2020.
Occipital Brain Lobe | Function, Anatomy, Position, and Structure
The occipital lobe participates in vision processing. It processes and interprets everything we see. The occipital lobe is also responsible for analyzing contents, such as shapes, colors, and movement, and also for interpreting and drawing conclusions about the images we see.
Boundaries, Anatomy, Position, and Structure of the Occipital Brain Lobe
The boundaries of the occipital lobe include the edges of the parietal and temporal lobe.
The occipital lobe occupies the posterior parts of the hemispheres. On the convex surface of the hemisphere, the occipital lobe has no sharp boundaries separating it from the parietal and temporal lobes.
The exception is the upper part of the parietal-occipital groove, which, located on the inner surface of the hemisphere, separates the parietal lobe from the occipital lobe. The furrows and edges of the upper canopy of the occipital lobe are unstable and have a variable structure.
On the inner surface of the occipital lobe, there is a groove of spores, which separates the wedge (triangular norm of the occipital lobe) from the lingual gyrus and the occipital-temporal gyrus (1).
In the occipital lobe of the cerebral cortex is, the following fields are positioned:
- Area 17 – Gray matter buildup in a visual analyzer.
This field is the primary zone. It is made up of 300 million nerve cells.
- Area 18 – It is also a nuclear set of visual analyzers. This field performs the function of perceived writing and is a more complex secondary area.
- Area 19 – This field is involved in evaluating the value of what we see.
- Area 39 – This brain part does not completely belong to the occipital region. This area is located at the border between the parietal, temporal, and occipital lobes. Its functions include integrating visual, auditory, and general sensitivity of information (1).
The Function of the Occipital Brain Lobe
The function of the occipital lobe is related to the perception and processing of visual information, as well as the organization of complex processes of visual perception.
At the same time, the upper half of the retina, which detects light from the lower field of vision, is projected into a wedge; in the reed area, the gyrus is the lower half of the retina, noticing the light from the upper field of view(1).
In the occipital cortex, there is a primary visual area (the cortex of the part of the sphenoid gyrus and the lingual lobule). There is a local representation of the retinal receptors. Each retinal point corresponds to its portion of the visual cortex, while the yellow dot area has a relatively large representation area.
In connection with the incomplete intersection of visual pathways, the same half of the retina is projected into the visual area of each hemisphere. The presence of retinal projection of both eyes in each hemisphere is the basis of binocular vision.
The neurons of these zones are polymodal and respond not only to light but also to tactile and auditory stimuli. Different types of sensitivities are synthesized in this visual area, more complex visual images emerge and they are recognized.
An example can be given to understand the function of the occipital lobe in visual perception. When we look at a map and “put” the route planner information into our working memory, the first step would be to process millions of light stimuli in the recognition centers, i.
Furthermore, the occipital lobe receives incoming information, which is processed and immediately sent to the hippocampus, where it is formed into memory. Firstly, it becomes short-term memory. Accordingly, we remember the name of the place of destination and remember it as we move along this route.
As we have already stated, the occipital lobe is responsible for the visual perception of information, as well as its operational storage. Generally, everything projected by the retina is recognized and formed into a specific image in the occipital lobe.
For absolutely healthy people, this proportion works independently and flawlessly, but irreparable consequences can occur with injuries and some illnesses. Sometimes, total blindness can occur. This is the process that happens if there is a damage on the surface of the primary visual cortex.
Light signals transmit information to the occipital lobe via nerve endings, which represents a form of irritation or stimuli for the retina. The nerves then transmit information to the diencephalon, another part of the brain. And diencephalon, in turn, sends information to the primary visual cortex, called the sensory cortex.
From the primary sensory cortex, nerve signals are sent to the adjacent areas and are called sensory associative cortex areas. The main function of the occipital lobe is to send signals from the primary visual cortex to the visual associative cortex. The areas described together analyze the visual information observed and retain visual memories.
As already implied, this occurs when the primary visual cortex, whose surface is visible, is damaged. Complete damage to the primary cortex occurs in three cases, as a result of a head injury, as a result of the development of a tumor on the surface of the brain, and finally, yet very rarely, as a consequence of certain congenital anomalies.
Damage of the Occipital Brain Lobe
Damage to the primary visual cortex leads to a form of central blindness called the Anton’s syndrome; patients cannot recognize objects via their sense of sight and are completely unaware of their deficits (2).
Epileptic seizures in the area of the occipital lobe cause visual hallucinations, most commonly in the form of dashes and a colored mesh that appears on the contralateral field of view.
Damage of the occipital brain lobe can occur as a result of a head injury, a tumor on the surface of the brain, and certain congenital anomalies.
However, focal lesions do not lead to complete loss of vision. For example, after taking a familiar object in his hands, a patient may describe the object he/she is touching. However, if that same object is shown in the picture, then the patient will not be able to describe its shape and color. In medical language, this condition is called visual agnosia.
At times, focal lesions can localize and restore vision and perception. However, it is important to note that the chances of a partial recovery in children are higher than those in patients whose brain is already formed and not growing anymore. Treatment is usually done surgically(2).
Pain in the occipital brain lobe region
There are many different causes of pain in this region. Some of them include:
- Nerve tension and stress. With prolonged tension, neck and back muscle spasms and neck pain occurs. Also, pain in the occipital brain region can be localized. The patient can diminish the pain by breathing calmly and deeply. If the pain does not stop after the patient feels relaxed, a visit to a doctor is obligatory.
- Osteochondrosis of the cervical spine. This condition results in sharp pain in the back of the head. Specialized forms of gymnastics can help. However, the patient must see a neurologist.
- High blood pressure. This condition can cause pain with a feeling of fullness. Pressure control is essential for extending one’s lifetime. Contact a neurologist if you feel pain in the occipital part of your brain and suffer from blood pressure disorders.
- Increased intracranial pressure. This serious condition is characterized by oppressive eye pain. The pain is localized in the occipital lobe. The patient must immediately see a doctor.
Conclusion
The occipital lobe is located in a triangle, the apex of which is the parietal lobe and the sides of the temporal lobes of the brain. The cerebellum is positioned below the occipital lobe. This brain part has a variable structure.
Its key function is processing visual information. The visual cortex, located on both hemispheres of the occipital lobe, provides binocular vision – the world seems vast and wide to the human eye.
The visual cortex, called the associative area, constantly communicates with other brain structures, forming a complete image of the world.
Thus, this or that visual image may be accompanied by negative emotions or vice versa: long-term visual memory may evoke positive emotions.
The occipital lobe, along with simultaneous signal analysis, also plays the role of an information container. However, the amount of such information is insignificant, and most of the environmental information is stored in the hippocampus.
The occipital cortex is strongly associated with feature integration. The essence of these theories is that the cortical analytic centers of separate properties of an object (color) are processed separately, and in parallel with the processing of other information.
To sum up, the occipital lobe is responsible for processing visual information and their integration into the general relation to the world; storing visual information; interaction with other areas of the brain, and, partly, tracking their functions; as well as the binocular perception of the environment.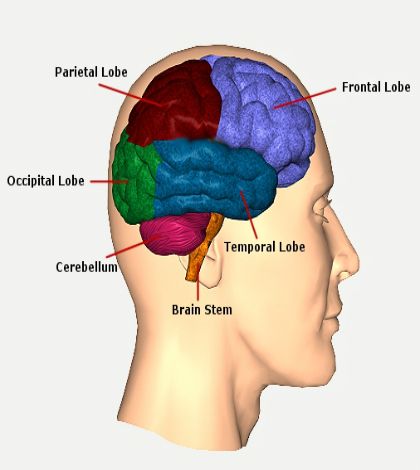
References:
- Rehman A, Al Khalili Y. Neuroanatomy, Occipital Lobe. [Updated 2019 Jul 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544320/ Found online at: https://www.ncbi.nlm.nih.gov/books/NBK544320/
- Macaskill J. A CASE OF OCCIPITAL LOBE INJURY. Br J Ophthalmol. 1945 Dec;29(12):626-8. PMID: 18170164; PMCID: PMC512175. Found online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC512175/
Speech development disorders in children and epileptiform activity on the EEG
Specific speech development disorders (SPP) according to the ICD-10 classification [1] are headings F80.1 – expressive speech disorder and F80.2 – receptive speech disorder. They include disorders in which speech suffers already in the early stages of the child’s development and the previous period of its normal development is not traced.
In clinical practice, expressive and mixed expressive-receptive variants of the pathology are most common, manifested by a significant delay in the development of expressive speech compared to the development of understanding, while expressive speech is characterized by significant deviations.
Speech in developmental dysphasia does not serve as a full-fledged means of communication, organization of behavior and individual development. Intellectual insufficiency and a limited stock of knowledge, observed in patients at different age periods, are thus of a secondary nature. This distinguishes patients with dysphasia from children with general mental retardation or mental retardation, which are characterized by a uniform incomplete formation of all higher mental functions and intellectual abilities.
Of great importance in the comprehensive examination of children with developmental dysphasia is electroencephalographic examination (EEG), which allows you to assess the level of morphological and functional maturity of various parts of the CNS (cerebral cortex and subcortical regulatory structures), which can be affected by pathological factors. In the course of neurophysiological studies, in a number of patients with developmental dysphasia, changes in the EEG were found [3, 6], in particular, a dysrhythmic type, a low index and disorganization of the α-rhythm and even its absence, a hypersynchronous type of EEG, a decrease in CNS reactivity, as well as focal changes in the frontal, fronto-temporal, fronto-parietal, temporal-parietal and occipital-parietal regions.
In foreign studies, much attention was paid [7-11] to the identification of the development of epileptiform changes on the EEG in patients with dysphasia, including the so-called benign epileptiform childhood discharges (BECD). These studies were influenced by the concept of congenital disorders of brain maturation developed by H. Doose et al. [12]. According to this concept, some patients have a genetically determined impairment of brain maturation in the prenatal period, which is the cause of a complex of pathological conditions: epileptic seizures, EEG-like patterns of DERD, and developmental disorders, in particular dysphasia and autistic disorders.
The purpose of this study was to evaluate the parameters of neurophysiological examination of children with developmental dysphasia according to EEG and EEG monitoring with the identification of epileptiform activity.
Material and methods
EEG recording was carried out in 65 children, 48 boys and 17 girls, with developmental dysphasia. Their age was from 3 to 4 years 11 months. 31 children had OHP level 1 and 34 – OHP level 2.
The examination of children was carried out on an outpatient basis, and at the time of the examination, the children did not receive any drug therapy.
Developmental dysphasia was diagnosed based on the ICD-10 criteria [1] as a developmental disorder of expressive speech (heading F80.
The study group excluded children whose speech development lag was due to hearing loss, mental retardation, autism, severe somatic pathology, malnutrition, and the influence of adverse social factors (insufficient communication and education).
After EEG changes were detected, in order to clarify their nature, in 27 cases out of 65, video-EEG monitoring was performed in the states of wakefulness and sleep.
When analyzing the EEG results, the method of visual assessment of the general pattern of electrical activity of the brain was used with the calculation of the index of severity and amplitude of the main rhythm (α-rhythm), topography, amplitude and representation of other frequency components, as well as the identification of asymmetry and foci of pathological activity. Together, this made it possible to attribute the EEG to a certain type according to the classification of E.
Results
Many parents of the examined children pointed out that already at an early age of the child they paid attention to the absence or insufficient expression of his babbling, silence. At the same time, they often emphasized that the child seemed to understand everything, but did not want to speak. Instead of speech, facial expressions and gestures developed, but the children used them selectively, mainly in emotionally charged situations. The first words and phrases appeared late. At the same time, parents noted that in addition to the lag in speech, in general, the children developed normally. Having a meager active vocabulary, the children used babbling words, sounds, and onomatopoeia. If elementary speech appeared, then many reservations were noted, to which the children paid attention and tried to correct the mistakes.
At the time of the survey, the volume of the active vocabulary (stock of spoken words) in children with OHP level 1 did not exceed 15-20 words, and with OHP level 2, it ranged from 20 to 50 words.
When evaluating the EEG in the majority – 47 (72.3%) patients, EEG type I was noted – organized, in 4 (6.2%) children the EEG approached the organized type according to a fairly clearly expressed α-rhythm with a high value of its index, but it did not quite correspond to it due to the increased representation of slow irregular oscillations, more often in the caudal areas. Therefore, in these cases, the EEG type was assigned to I-b. In 13 (20%) children, an EEG type was detected corresponding to or approaching IV (disorganized, with a predominance of α-activity) in terms of significantly represented diffuse irregular waves, but characterized by a high index (60-87%) of the α-rhythm. In 1 (1.5%) child, the EEG was classified as type III (desynchronous) due to the quantitative predominance of low-amplitude slow diffuse waves and a significantly reduced amplitude of rhythmic α-activity (less than 40 μV).
Age-appropriate frequency characteristics of the α-rhythm were present in 57 (87.7%) cases, slowing down of background activity – in 8 (12.3%) children. In the background EEG recording in the majority – 57 (87.7%) children, the predominance of the α-rhythm in the posterior sections of the cerebral cortex of the cerebral hemispheres was revealed, with an emphasis on the amplitude in the occipital region. In a smaller part of cases, in 8 (12.3%) children, a shift of the α-rhythm gradient to the parietotemporal regions was observed.
One of the features of the EEG, found in 8 (12.3%) of the examined children, were bursts of polymorphic slow activity and acute α-waves in the central-temporal (in 4 children) and fronto-central-anterotemporal (in 4) areas of the cerebral hemispheres brain. In their morphology, they were close to the central τ-rhythm and could be associated with immaturity or residual organic damage to the CNS.
When studying the response to rhythmic photostimulation with a frequency of 2.0 to 30.0 Hz, certain features were revealed: in 56 (86.1%) children, no response to rhythm assimilation was found at a frequency of photostimulation from 2.0 to 30.0 Hz ; in 4 (6.2%), a pronounced reaction of rhythm assimilation was registered with low-frequency photostimulation (6; 8 Hz), in 5 (7.7%) – with relatively high-frequency photostimulation (10 Hz).
B tab. 1 summarizes the changes detected on the EEG. It should be noted that in 8 (12.3%) children with developmental dysphasia, the presence of specific epileptiform patterns was registered, despite the absence of epileptic seizures in their anamnesis, at the time of the examination and for the entire period of dynamic observation.
All patients who were found to have epileptiform discharges on a routine EEG underwent video-EEG monitoring in the waking and sleeping states to exclude the diagnosis of epilepsy, including latent seizures, as well as the phenomenon of electrical status epilepticus in the non-REM sleep phase – ESES ( electrical status epilepticus during slow sleep).
First of all, let’s pay attention to the DERD, which is a specific epileptiform pattern with a characteristic morphology – a high-amplitude five-point electric dipole, consisting of sharp and slow waves; the initial component consists of a three-phase sharp wave, which always exceeds the subsequent negative slow wave in amplitude. FERD low index were found in 3 (4.6%) children. They were localized in 1 patient in the left central-temporal region, in 1 patient in the right temporal region, and in 1 patient in the occipital region of the brain. In another 5 (7.7%) children, low-index epileptiform discharges were found, the representation of which did not correspond to the picture of epileptic encephalopathy and the ESES pattern.

Features of EEG changes were also analyzed depending on the severity of OHP (Table 2) . It should be noted right away that no statistically significant differences between the groups of patients with ONR levels 1 and 2 were found in any of the EEG characteristics. Nevertheless, there was a trend towards an increase in the frequency of EEG changes in children with more pronounced speech impairments at level 1 ONR. Thus, when determining the frequency characteristics of the α-rhythm, a slowdown in background activity was more often found among children with ONR of the 1st level (16.1%) than of the 2nd level (8.8%). Epileptiform activity was also detected more often in OHP of the 1st level – in 5 (16.1%) patients than in the 2nd – in 3 (8.8%). In particular, DERD was noted in 2 (6.5%) children with OHP level 1 and 1 (2.9%) with OHP level 2. Complexes of an acute-slow wave of a low index were determined in 3 (9.7%) children with ONR of the 1st level and 2 (5.9%) with ONR of the 2nd level.
All children whose EEG revealed a slowdown in background activity of the 1st-2nd degree, as well as epileptiform activity, were monitored by EEG at intervals of 4-6 months for 1.5-3 years during follow-up. At the same time, the frequency characteristics of the background activity approached the age norms of the main rhythm, and the organization of the rhythm increased. This suggests that the changes described above are of a transient nature, reflecting manifestations of the functional immaturity of the brain. The study of epileptiform activity over time showed that it remained subclinical, and there were no signs of its progression.
Discussion
As the results show, not all children with developmental dysphasia have changes in the bioelectrical activity of the brain, and the nature of these changes does not always correspond to the severity of speech disorders.
EEG epileptiform activity was detected in 8 (12.3%) patients with developmental dysphasia, but in all cases it was not clinically manifested. In 3 (4.6%) children, epileptiform activity was determined in the form of DERD, in 5 (7.7%) – epileptiform discharges with a low index. Attention is drawn to cases of developmental dysphasia with epileptiform activity, which has frontal and temporal localization in the dominant hemisphere (in right-handers – left, in left-handers – right). The temporal lobe – Wernicke’s area – field 22 of the cortex of the left hemisphere, is responsible for the perception and differentiation of auditory signals, complex processes of understanding speech, and the inferior frontal gyrus (Broca’s area – fields 44 and 45) provides a program of speech utterance and the motor side of speech.
Local changes in the baseline EEG observed in some children with developmental dysphasia correspond to the assumption that the speech disorders observed in them are based on dysfunction of the frontal and temporal lobes of the cerebral hemispheres. At the same time, the opinion of a number of researchers that children with developmental dysphasia may be involved in the pathological process of both the left and right hemispheres of the brain should be recognized as justified. According to C. Njokiktien [4], when recording EEG in a group of 163 children with developmental dysphasia, local (not only epileptiform) disorders were detected in ¼ of cases, of which 11 patients – in the leads on the right, in 25 – on the left, in 6 – at both sides. Apparently, the localization and nature of CNS damage, which impede the normal formation of lateralization of speech functions, prevent compensatory restructuring and exacerbate the difficulties in mastering speech in children.
It should be noted that in our observations, epileptiform activity was more often detected in ONR of the 1st level – in 5 (16.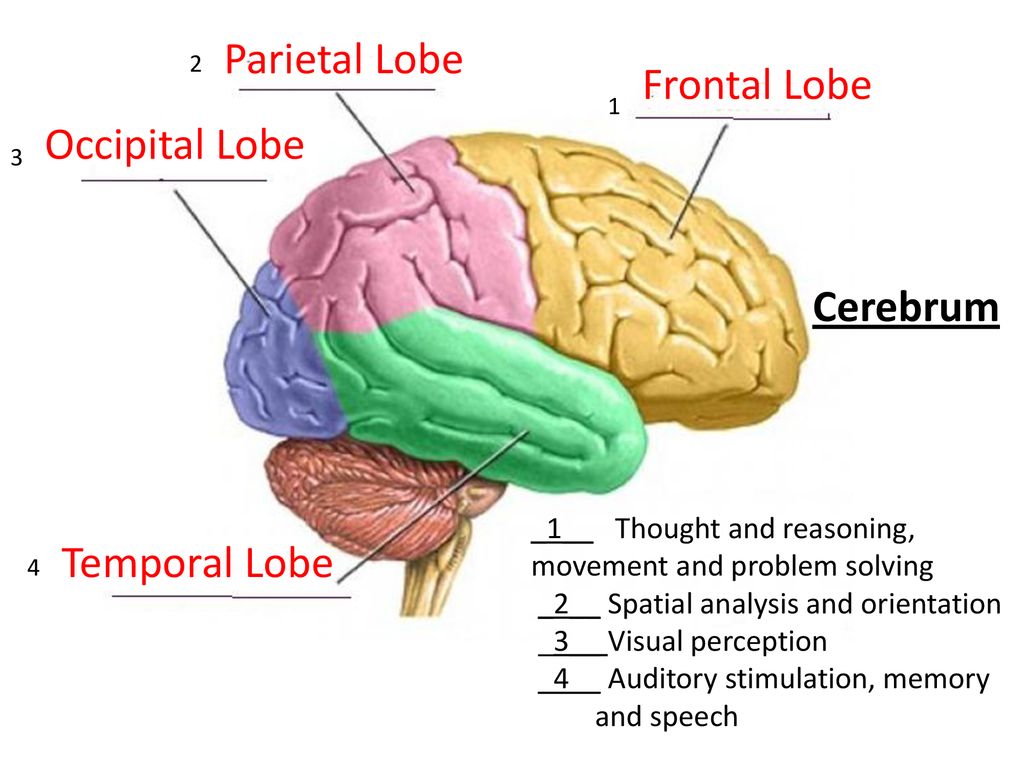
H. Doose et al. [12] consider DERD on the EEG as a genetically determined epileptiform phenomenon with an autosomal dominant mode of inheritance, low penetrance, and variable gene expression. This epileptiform activity in most cases is not associated with structural organic damage to the brain, it is age-dependent. As the child grows older and the CNS matures, FERD completely regresses to adolescence [17, 18]. However, despite the term “benign”, this epileptiform activity can have a negative impact on the development of higher mental functions [19, twenty].
In a population of healthy children, according to H. Doose [18], epileptiform activity on the EEG occurs in approximately 2% of cases. In a study by O. Eeg-Olofsson et al.
Among the epileptic syndromes that need to be excluded in speech disorders in children using EEG, the literature considers epilepsy with ESES or prolonged peak-wave complexes in non-REM sleep (CSWS), as well as Landau-Kleffner syndrome [2, 4].
ESES is an EEG pattern that is detected in children 4-5 years of age and older, in some cases there are indications of early CNS lesions and various neurological disorders in the anamnesis.
In Landau-Kleffner syndrome – “acquired epileptic aphasia” – there is a rapid disintegration of speech understanding (auditory verbal agnosia). Aphasia usually develops between the ages of 3-7 years. A previously normally developing child loses the ability to understand speech addressed to him and to speak. In some cases, loss of speech occurs gradually and can stretch over time up to six months, but more often it happens suddenly. EEG changes are detected in 100% of cases, characterized by pronounced paroxysmal activity – often in the form of spike-wave complexes, multiple sharp waves with a predominance in the temporal regions on one or both sides, usually asymmetric.
When epileptiform changes are detected on the EEG, patients with developmental dysphasia are shown to conduct video-EEG monitoring in the states of wakefulness and sleep, followed by periodic monitoring of the EEG in dynamics.
As noted by L. Neuschlova et al. [9], the frequency of detection of epileptiform activity among patients with developmental dysphasia varies so widely in studies of different authors (from 9 to 94%) that a real assessment of its occurrence is difficult. This is due to methodological differences, including the criteria for diagnosing dysphasia, the inclusion or exclusion of patients with autistic disorders, epileptic syndromes or a history of epileptic seizures, as well as approaches to EEG recording and principles for assessing epileptiform activity.
When performing EEG or EEG monitoring C. Duvelleroy-Hommet et al. [7] identified DERD in 38% of 24 children with developmental dysphasia, A. Picard et al. [10] – in 50% of 52, B. Echenne et al. [8] – in 93% of 32, L. Neuschlova et al. [9] – in 39% of 28 patients. At the same time, a significant number of children suffering from epilepsy and autism, but also having a pronounced lag in speech development, were not excluded from the examined groups, which, of course, led to an increase in the frequency of detection of DERD. At the same time, R. Tuchman et al. [11] noted epileptiform activity in 58% of children with dysphasia and epilepsy, but only in 9% – without epilepsy.
Our data allow us to clarify the frequency of occurrence of epileptiform activity in patients with developmental dysphasia at the age of 3-4 years without autistic manifestations and epileptic seizures in history; it was shown that epileptiform activity is more often detected with a more pronounced lag in speech development, corresponding to the OHP of the 1st level (16.
Complex therapy is indicated for children with developmental dysphasia to overcome speech disorders; along with carrying out speech therapy and psychological and pedagogical correction, it is recommended to prescribe repeated courses of nootropic drugs. When choosing drug therapy in cases of a combination of developmental dysphasia with subclinical epileptiform activity on the EEG, preference should be given to nootropic drugs that do not cause its increase. Such a drug is pantogam (hopantenic acid), the positive effect of which has been confirmed not only in developmental dysphasia in children [24], but also in the treatment of speech, cognitive and behavioral disorders in the group of patients with epilepsy at the age of 3-4 years, among which none in one case, no negative EEG dynamics was recorded [25].
Summarizing the data presented in the article, it can be noted: 1) when conducting EEG studies, 12.3% of children with developmental dysphasia had signs of epileptiform activity that did not manifest themselves clinically. Epileptiform activity was detected more often in OHP level 1 (16.1% of patients) than in OHP level 2 (8.8%), i.e. its frequency depended on the degree of retardation in speech development; 2) when epileptiform changes on the EEG are detected, patients with developmental dysphasia are shown to conduct video-EEG monitoring in the states of wakefulness and sleep, followed by periodic monitoring of the EEG under the supervision of a neurologist in dynamics. It is necessary to conduct a differential diagnosis in order to exclude rare epileptic encephalopathies, including ESES and Landau-Kleffner syndrome.
Localization and lateralization signs – Epilepsy – Directory of nosologies – List of nosologies
Hemispheric lateralization
Specific features of a focal seizure are useful in determining the onset of an attack or network in one hemisphere.
Features that suggest seizure lateralization are described below. They provide strong evidence of lateralization, and sometimes they can be falsely lateral.
- Unilateral ictal clonic activity or ictal dystonia suggests lateralization of the attack in the contralateral hemisphere.
- An early forced version of the head suggests lateralization in the hemisphere opposite to the direction of the head, i.e. if the head turns to the right, the start of capture is in the left hemisphere.
- Ictal speech is lateral in the non-dominant hemisphere.
- Ictal aphasia is lateral in the dominant hemisphere.
- Postictal dysphasia lateral to the dominant hemisphere.
- Retained awareness during ictal automatisms is lateral to the non-dominant hemisphere.
- Postictal wiping of the nose lateral to the hemisphere ipsilateral to the hand used to wipe the nose.
- Unilateral blinking of the eyes is lateralized to the hemisphere ipsilateral to that eye.
- Ictal vomiting is located in the non-dominant hemisphere.
Shared localization
Frontal lobe attacks
Frontal lobe – attacks have distinctive features depending on the area of the frontal lobe. Seizure types range from focal hyperkinetic seizures with pelvic movements and pedaling to focal bilateral motor seizures with an asymmetric tonic set. Frontal lobe seizures may begin with a short aura, even if the seizures occur from sleep. Seizures are usually short and may have prominent vocalizations, bizarre behavior, urinary incontinence, and head and eye deviation. Frontal lobe attacks can be exclusively nocturnal and often clustered. The ictal EEG may not show ictal patterns or may be masked by motion artifact.
Nocturnal spasms of the frontal lobe may be mistaken for parasomnias, however:
- Frontal lobe seizures are usually short events (less than 2 minutes), with stereotypic features seen with each seizure and awareness.
Parasomnias are usually longer (more than 10 minutes), have variable features from event to event, and are characterized by a confused state where the patient does not remember the event afterwards.
- Clustering is rare in parasomnias, and the most frequent non-SEM parasomnias usually occur 1–2 hours after falling asleep in the first cycle of deep slow sleep. Attacks of the nocturnal frontal lobe usually occur throughout the night, and most often half an hour after falling asleep or waking up.
Frontal seizure subtypes
Primary sensorimotor cortex
Seizures are focal motor seizures characterized by localized clonic, tonic-clonic, tonic, or myoclonic activity. They can manifest as a Jacksonian march, where unilateral tonic-clonic movements begin in one muscle group and gradually spread to adjacent groups, reflecting the spread of ictal activity through the motor cortex in accordance with the homunculus. There may be focal sensorimotor features, such as unilateral tingling, or in combination with motor features.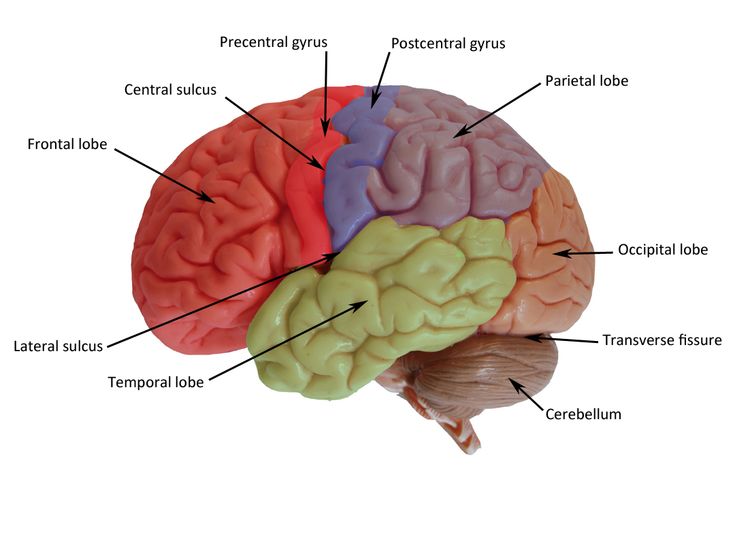
Supplementary sensorimotor cortex
Seizures are focal bilateral motor seizures characterized by abrupt onset and asymmetrical tonic posture lasting 10–40 seconds with minimal post-seizure confusion. There is an asymmetric position of the upper limbs with straightening of the upper limb of the contralateral hemisphere and flexion of the ipsilateral upper limb. Loud vocalization or speech arrest may occur when seizures occur. The head and eyes often turn in the direction of an attack contralateral to the hemisphere. A focal somatosensory seizure may occur before the onset of a motor seizure.
Orbitofrontal cortex
Decreased awareness, initial repetitive automatisms, olfactory hallucinations, illusions and autonomic manifestations are observed.
Frontopolar cortex
Seizures are characterized by violent thoughts, reduced awareness, an ipsilateral version of the head and eyes with possible extension to the opposite side, autonomic signs, and axial tonic-clonic movements leading to falls.
Dorsolateral frontal cortex
In the dominant hemisphere, an attack occurs in or near Broca’s area, may lead to aphasia or dysphasia in the patient, who then wakes up and responds. Motor signs are encountered, most often tonic, accompanied by a contralateral version of the head and eyes. There may be violent thoughts.
Cingular bark
Seizures are characterized by automatisms from the very beginning with a weakened awareness, features of emotions and mood, vegetative signs. Focal emotional attacks with laughter (gelastic) may occur.
Frontoparietal operculum
Seizures are characterized by facial (mouth and tongue) clonic movements (which may be unilateral), laryngeal symptoms, difficulty articulating, swallowing or chewing, and hypersalivation. Autonomic (eg, epigastric, urogenital, gastrointestinal, cardiovascular, or respiratory) and emotional (eg, fear) symptoms are common. Taste hallucinations are especially common.
Seizures of the temporal lobe
Seizures of the temporal lobe are characterized by slowing down of behavior and impaired awareness. Automatisms are common during an attack and include oral and/or manual automatisms. There may be sensory (auditory), emotional (fear), cognitive (déjà vu), or autonomic features (epigastric sensations, tachycardia, discoloration) at the onset of an attack before awareness is impaired. The occurrence of post-attack confusion is typical.
Specific features suggest the onset of the attack in the dominant or non-dominant temporal lobe. Ictal speech, spitting, vomiting, drinking, the urge to urinate, and unconscious automatisms suggest the onset of seizures in the non-dominant temporal lobe. Postictal speech impairment suggests an attack in the dominant temporal lobe. Dystonia of the upper extremity is a useful lateralizing sign that defines an attack from the contralateral hemisphere. In contrast, manual automatisms usually occur on the ipsilateral side.
In infants, temporal seizures may be mild and present with pallor, apnea, and slowness of behaviour. There may be earlier and more pronounced motor manifestations, including tonic seizures and epileptic spasms, which may reflect different forms of spreading activity in the developing brain.
Temporal focal seizures with reduced awareness may have similar features to frontal focal seizures with impaired awareness, however, impaired awareness of temporal origin tends to have a slower onset and progression, and posttictal confusion is more pronounced.
Temporal focal seizures with reduced awareness must be distinguished from absence seizures. While both may be automatic, temporal lobe seizures are usually longer (more than 30 seconds), and are accompanied by postictal confusion.
Temporal lobe seizure subtypes
Mesial temporal lobe including hippocampus
Seizures originating in the mesial temporal lobe are characterized by distinctive onset features such as an autonomic seizure with increasing epigastric sensation or abdominal discomfort, or a cognitive seizure with déjà vu/jame vu, or an emotional seizure with fear.
Lateral/neocortical temporal lobe
Seizures of the lateral temporal lobe may begin as a focal seizure with auditory sensations or vertigo.
Seizures of the parietal lobe
Parietal onset seizures can be difficult to diagnose, especially in children, due to the subjective nature of the sensations experienced. Positive and/or negative sensory experiences occur. Paresthesia is usually reported, but disorientation, complex visual hallucinations, dizziness, and visual illusions and impaired body sensation (somatic illusion) may occur.
Parietal lobe seizure subtypes
Primary sensory area (postcentral gyrus)
Seizures begin with a contralateral (or less commonly ipsilateral or bilateral) focal somatosensory seizure, most commonly paraesthesia with tingling and/or numbness. Stinging, tickling, crawling, or electric current sensations may appear in the affected part of the body. Sensory sensation can spread sequentially along a part of the body, the attack spreads through the cortex in accordance with the sensory homunculus (Jackson’s march), while motor activity in the affected part of the body usually remains. Rarer sensory sensations include pain and heat perception (eg, feeling hot or cold).
Non-dominant parietal cortex
Seizures may be characterized by distortions in body sensation with sensation of movement (eg, swimming) or change in position (eg, twisting) in a stationary limb.
Secondary sensory area (upper wall of the sylvian sulcus)
There are focal cognitive seizures followed by a feeling of being unable to move, successively spreading through the body parts (ictal paralysis), this may be followed by clonic twitching in the affected body parts.
Parieto-occipital junction
There are focal cognitive seizures with visual illusions, including macropsia (objects in the visual field are enlarged) or micropsia (objects appear smaller). Versive eye movements (usually contralateral) or epileptic nystagmus are observed. If nystagmus is seen, usually with a fast component towards the contralateral hemisphere of the onset of the attack with a slow component returning to the ipsilateral side.
Paracentral lobule
Seizures occurring in the non-dominant hemisphere are characterized by sexual sensations affecting the genitals. The next phase of the attack may be accompanied by sexual behavior.
Dominant parietotemporal region
Observed focal cognitive seizures are characterized by speech difficulties with difficulties in reading, calculating and writing.
Frontoparietal operculum
Seizures are characterized by facial (mouth and tongue) clonic movements (may be unilateral), laryngeal symptoms, difficulty articulating, swallowing or chewing, and hypersalivation. Autonomic (eg, epigastric, urogenital, gastrointestinal, cardiovascular, or respiratory) and emotional (eg, fear) features are common. Taste hallucinations are especially common.
Seizures of the occipital lobe
Seizures originating in the occipital lobe are characterized by focal sensory visual seizures, which are subjective sensations that make diagnosis difficult in young children.
Seizure subtypes of the occipital lobe
Primary visual cortex
Seizures in this area result in focal sensory visual seizures, which can be positive visual effects (usually multicolored shapes such as circles, flashes) or negative effects such as loss of part of the visual field or blindness (amaurosis). Bilateral vision loss may occur and it may be black or white. More complex visual imagery, which are considered focal cognitive seizures, is not observed in seizures that occur in this area. The visual phenomenon is manifested in the contralateral field of view in the hemisphere of the onset of the attack. If positive visual phenomena occur in part of the visual field, the person may look in that direction during an attack. It is helpful to ask the child to draw what he sees during this attack.
Extrastriate bark
Seizures in this area are associated with more complex formed visual hallucinations such as images of people, animals, or scenes and are considered focal cognitive seizures.
Parieto-occipital junction
Epileptic nystagmus can be seen, usually with a fast component opposite the hemisphere of the onset of the attack, and a slow component returning to the ipsilateral side. Eye movements are usually conscious and may be accompanied by a version of the head or torso. Fluttering of the eyelids or forced closing of the eyes may also occur.
Occipital cortex below the spur groove
Occipital seizures originating in this area tend to spread to the temporal lobe resulting in a focal seizure with reduced awareness
Occipital cortex above the spur groove
Occipital seizures originating in this area may extend to the parietal lobe, fronto-parietal operculum, or frontal lobes.
Seizures of unknown onset
For everyday purposes, seizures are broadly classified as having generalized or focal onset, these terms may be used where appropriate, but there are seizures that cannot be classified in this way and are classified as having unknown onset. Seizures of unknown onset may be further classified as either motor (eg, epileptic spasm, tonic-clonic) or non-motor (eg, behavioral arrest).
Unclassified seizure onset
Seizures may not be classified due to inadequate information to qualify the category with focal, generalized, or unknown onset. This can happen if it was not witnessed at the beginning, and if the results of studies (for example, EEG and imaging) are not yet available.
Epileptologist’s consultation in Moscow | Prices for consultation of neuropsychological diseases and disorders in children at Epihelp
The main diagnostic methods for epilepsy are magnetic resonance imaging and electroencephalogram.
An encephalogram is a method for studying the bioelectrical activity of the brain. This study allows you to determine the activity of neurons in various parts of the brain, the presence of pathological patterns (discharges) that indicate pathology. The harmlessness of diagnostics allows it to be widely used in childhood. Based on the results of the study, epileptiform activity, indications for MRI and the direction of the course of further treatment are determined.
EEG epileptiform activity in a child – what does it mean? The term epileptiform activity is understood as electrical oscillations recorded on the EEG in the form of sharp waves and peaks that differ from the total activity by more than 50%. The presence of epileptiform activity on the EEG may indicate the presence of epilepsy.
Indications for a study in children
Assign a study to diagnose various neurological and psychiatric diseases in children.
- Speech developmental delay: differential diagnosis between dysarthria (disturbance in the speech apparatus) and pathology of the speech centers of the brain.
- Different types of epilepsy, from generalized to myoclonus in certain muscle groups.
- Tics: to exclude the central genesis of the disease and disturbances in the electrical activity of the brain.
- Autism, behavioral disorders of the child (aggressiveness, etc.), attention deficit hyperactivity disorder.
- Enuresis or bedwetting.
- Sleep disorders, including somnambulism (sleepwalking): it is necessary to conduct an EEG during a night’s sleep.
- Brain injuries (concussions, bruises, and so on) – to identify foci of abnormal bioelectrical activity.
- If an oncopathology of the child’s brain is suspected, the lesion will show pathological signals, while it may not yet be visualized on an MRI.
- Frequent headaches with no explanation.
- Cognitive impairment: poor memory, poor school performance, attention deficit, excessive distraction, and so on.
How is the study carried out?
Electrodes are installed on the scalp in the projection of various parts of the brain, there are usually 19 of them, they are attached symmetrically on both sides of the head and in the center, a “cap” can be immediately put on, without the need to attach each electrode separately. Also, an ECG sensor is attached to the chest area, often additional sensors (myographic) are needed. At the same time, the child leads a normal life: walks, eats, plays sedentary games (mosaics, dolls, and others), and smart technology captures the activity of the baby’s brain.
The day before, the mother needs to prepare the child for the examination:
- Wash the hair, as excess sebum breaks the tight contact of the electrode with the scalp and distorts the result of the examination.
- Remove jewelry (earrings, barrettes, piercings).
- If the child is very small or shows aggression, is too restless, then it is recommended to carry out premedication, which is sedatives.
- Sometimes sleep deprivation is recommended, which is carried out in order to increase the information content of the study.
- It is not recommended to feed the child with energy products on the eve of the test: chocolate, strong tea, coffee, energy drinks, and so on.
- Warn the doctor of functional diagnostics about all medicines that the child receives in detailed dosages and frequency of use.
How is it deciphered and what can be seen?
Deciphering the EEG parameters of the brain in children takes quite a lot of time. Usually results are issued in a few days. Since electrical indicators from all leads are analyzed, all peaks and waves, their synchronism, symmetry are evaluated.
Parents are given a conclusion, a printout of the fragments of the record selected by the doctor and, in specialized centers, a disk with a record of the entire study.
It will not be possible to independently understand how to decipher the EEG of the brain in children, even with a very strong desire. Only a specialist can decipher the waves of electrical activity, especially in children, in whom even the norm has many variations, depending on the age of the child.
It is customary to distinguish the following main rhythms of electrical activity on the EEG:
- Alpha rhythm (or alpha rhythm precursor in children under 5 years of age). It is recorded at rest, in which the child sits or lies with his eyes closed and does nothing.
- Beta rhythm. It is detected with maximum concentration of attention: fast waves indicate active wakefulness.
- Theta rhythm. In a normal picture, the EEG in healthy children aged 2-8 years is one of the main rhythms, it is a wave, slightly exceeding the alpha rhythm in amplitude. The appearance of such indicators at an older age may indicate a mental retardation, a genetic consultation may be required.
Also, when deciphering the EEG in children, the synchronism of electrical potentials in both hemispheres is assessed. Disruption of synchronization indicates the presence of a pathological focus. It can be represented by a tumor, an epileptic focus, a vascular malformation, and so on.
Registration of epileptiform patterns is an important part of the study. Benign epileptiform patterns of childhood are now considered as a variant of the norm in the absence of epileptic seizures and regression in the development of the child.
With multiple discharges on the EEG, it is necessary to evaluate the clinic, it may be necessary to consult a baby with a psychologist and psychiatrist. It is necessary to decipher such results and make a diagnosis taking into account additional research methods.
Should we trust the study?
Encephalogram is a functional research method, so the results often largely depend on the child’s condition at the time of the examination.
Of course, if serious deviations are found, with the formation of foci of abnormal activity, indicating the presence of an epileptic focus, additional examination is required. The electrical method will only indicate the approximate localization of the focus (brain lobe). The most accurate localization of the process and the possible cause (vascular, neoplastic, atrophy due to intrauterine oxygen starvation of the brain, and so on) can only be determined using neuroimaging methods, primarily MRI.
How often should the test be repeated?
If the diagnosis did not show significant changes in brain electrical activity, then a second study in the absence of new symptoms can be omitted.







 [PubMed: 3603599]
[PubMed: 3603599] [PubMed: 32491402]
[PubMed: 32491402]



 This field is the primary zone. It is made up of 300 million nerve cells.
This field is the primary zone. It is made up of 300 million nerve cells.

 Parasomnias are usually longer (more than 10 minutes), have variable features from event to event, and are characterized by a confused state where the patient does not remember the event afterwards.
Parasomnias are usually longer (more than 10 minutes), have variable features from event to event, and are characterized by a confused state where the patient does not remember the event afterwards.