Absorbing and reflecting light: Light absorption – WikiLectures
Light Absorption, Reflection, and Transmission
We have previously learned that visible light waves consist of a continuous range of wavelengths or frequencies. When a light wave with a single frequency strikes an object, a number of things could happen. The light wave could be absorbed by the object, in which case its energy is converted to heat. The light wave could be reflected by the object. And the light wave could be transmitted by the object. Rarely however does just a single frequency of light strike an object. While it does happen, it is more usual that visible light of many frequencies or even all frequencies is incident towards the surface of objects. When this occurs, objects have a tendency to selectively absorb, reflect or transmit light certain frequencies. That is, one object might reflect green light while absorbing all other frequencies of visible light. Another object might selectively transmit blue light while absorbing all other frequencies of visible light. The manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. In this section of Lesson 2 we will discuss how and why light of certain frequencies can be selectively absorbed, reflected or transmitted.
Visible Light Absorption
Atoms and molecules contain electrons. It is often useful to think of these electrons as being attached to the atoms by springs. The electrons and their attached springs have a tendency to vibrate at specific frequencies. Similar to a tuning fork or even a musical instrument, the electrons of atoms have a natural frequency at which they tend to vibrate. When a light wave with that same natural frequency impinges upon an atom, then the electrons of that atom will be set into vibrational motion. (This is merely another example of the resonance principle introduced in Unit 11 of The Physics Classroom Tutorial.) If a light wave of a given frequency strikes a material with electrons having the same vibrational frequencies, then those electrons will absorb the energy of the light wave and transform it into vibrational motion.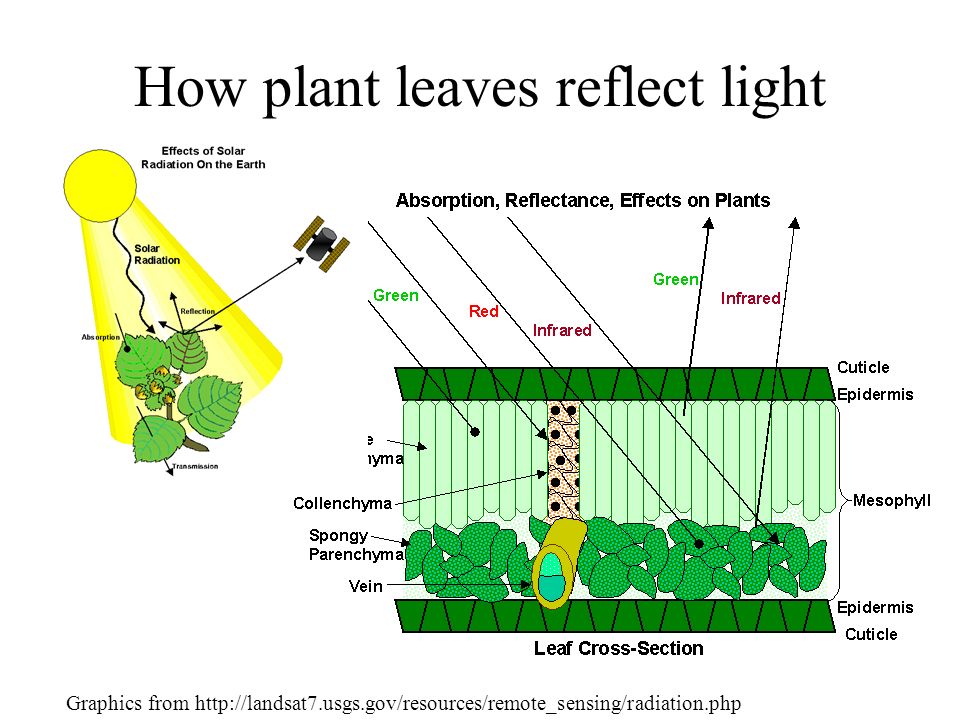
Visible Light Reflection and Transmission
Reflection and transmission of light waves occur because the frequencies of the light waves do not match the natural frequencies of vibration of the objects. When light waves of these frequencies strike an object, the electrons in the atoms of the object begin vibrating. But instead of vibrating in resonance at a large amplitude, the electrons vibrate for brief periods of time with small amplitudes of vibration; then the energy is reemitted as a light wave.
Where Does Color Come From?
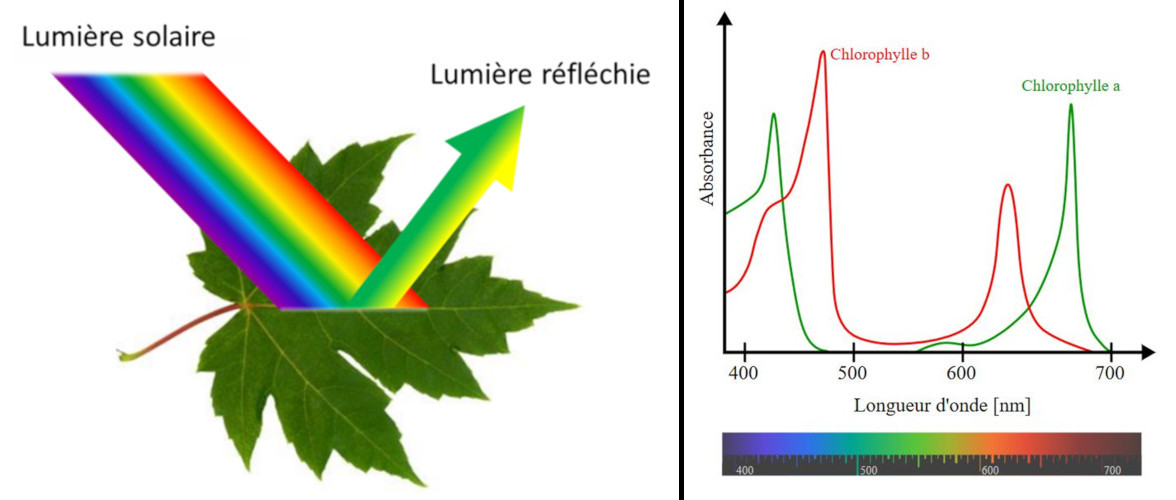
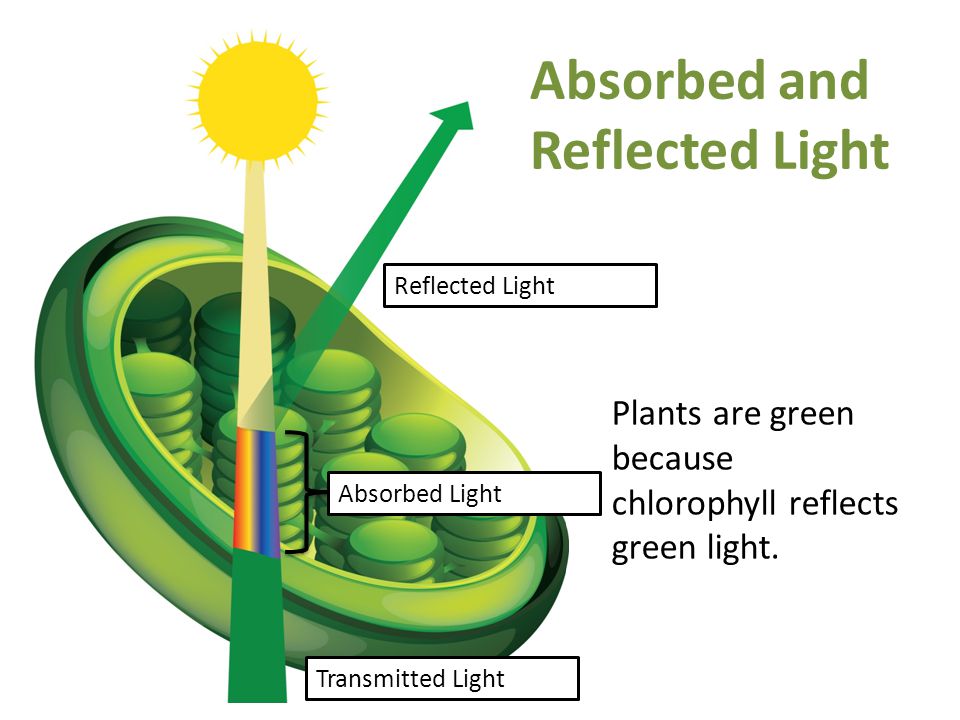
The color of the objects that we see is largely due to the way those objects interact with light and ultimately reflect or transmit it to our eyes. The color of an object is not actually within the object itself. Rather, the color is in the light that shines upon it and is ultimately reflected or transmitted to our eyes. We know that the visible light spectrum consists of a range of frequencies, each of which corresponds to a specific color.
Consider the two diagrams below. The diagrams depict a sheet of paper being illuminated with white light (ROYGBIV).
Check your understanding of these principles by determining which color(s) of light are reflected by the paper and what color the paper will appear to an observer.
Transparent materials are materials that allow one or more of the frequencies of visible light to be transmitted through them; whatever color(s) is/are not transmitted by such objects, are typically absorbed by them.
Express your understanding of this principle by filling in the blanks in the following diagrams.
The colors perceived of objects are the results of interactions between the various frequencies of visible light waves and the atoms of the materials that objects are made of. Many objects contain atoms capable of either selectively absorbing, reflecting or transmitting one or more frequencies of light. The frequencies of light that become transmitted or reflected to our eyes will contribute to the color that we perceive.
We Would Like to Suggest …
Sometimes it isn’t enough to just read about it. You have to interact with it! And that’s exactly what you do when you use one of The Physics Classroom’s Interactives.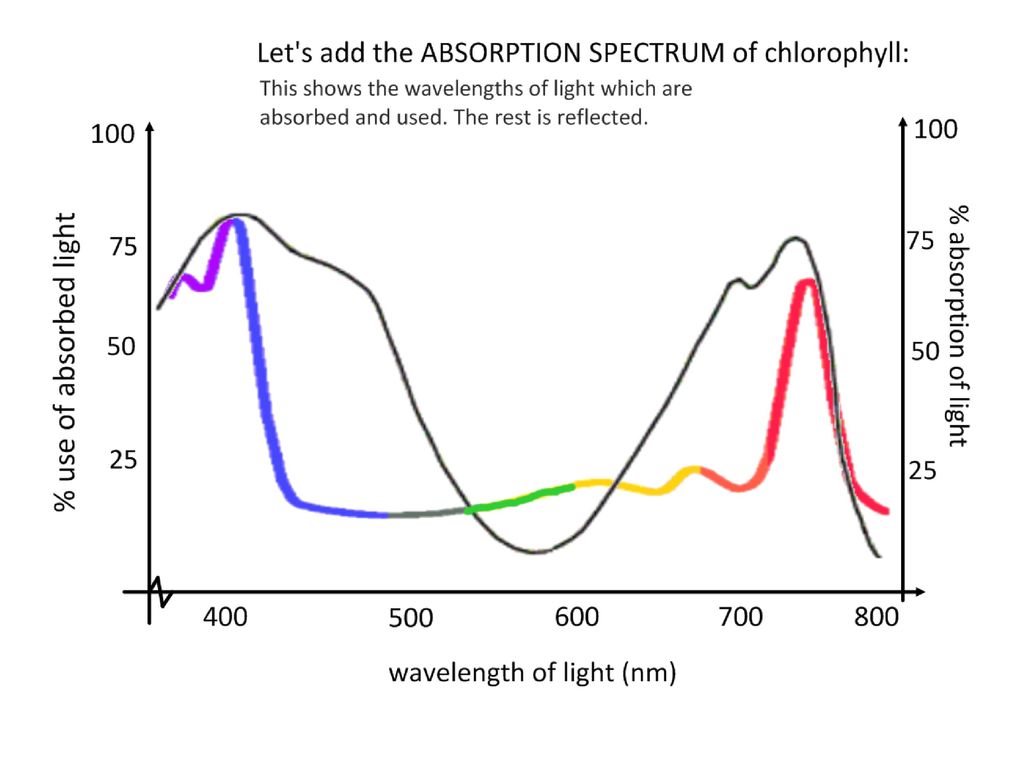
Visit: Stage Lighting Interactive
Check Your Understanding
1. Natural philosophers have long pondered the underlying reasons for color in nature. One common historical belief was that colored objects in nature produce small particles (perhaps light particles) that subsequently reach our eyes. Different objects produce different colored particles, thus contributing to their different appearance. Is this belief accurate or not? __________________ Justify your answer.
2.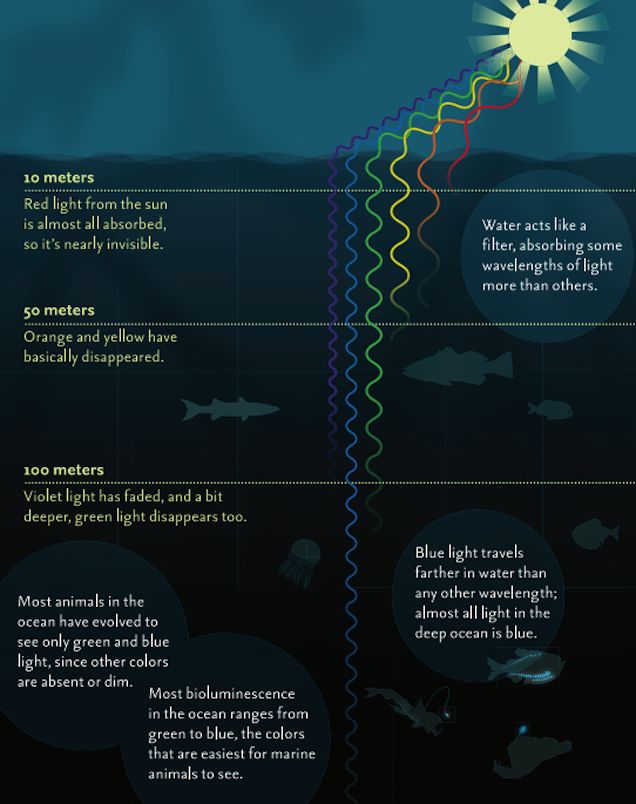
3. The diagrams depict a sheet of paper being illuminated with white light (ROYGBIV). The papers are impregnated with a chemical capable of absorbing one or more of the colors of white light. In each case, determine which color(s) of light are reflected by the paper and what color the paper will appear to an observer.
4. The appearance of a transparent object is dependent upon which color(s) of light is/are incident upon the object and which color(s) of light is/are transmitted through the object. Express your understanding of this principle by determining which color(s) of light will be transmitted and the color that the paper will appear to an observer.
Next Section:
Jump To Next Lesson:
Absorption / reflection of sunlight
What is the absorption and reflection of sunlight?
The Sun provides the Earth with most of its energy.
Earth’s surfaces are better at absorbing solar radiation than air, especially surfaces that are dark in color. You can feel this on a cold winter day when the sunshine warms your face and the air around you remains cold. Your skin and your clothes also absorb solar radiation and convert it to heat. If you wear a black jacket, it will absorb more radiation and make you feel warmer than if you wear a white or light-colored jacket.
The Earth is unevenly heated because it is a sphere.
Because Earth is a sphere, not all part of the Earth receives the same amount of solar radiation. More solar radiation is received and absorbed near the equator than at the poles. Near the equator, the Sun’s rays strike the Earth most directly, while at the poles the rays strike at a steep angle. This means that less solar radiation is absorbed per square cm (or inch) of surface area at higher latitudes than at lower latitudes, and that the tropics are warmer than the poles. This temperature difference shapes global atmospheric and ocean circulation patterns. Additionally, Earth’s tilt affects how much sunlight is received and absorbed by different parts of the Earth at various times of the year, and is why we experience the seasons. The amount of solar radiation received and absorbed also influences process in the biosphere by directly affecting plants and other organisms that photosynthesize and are the primary food source in most ecosystems (see species interactions).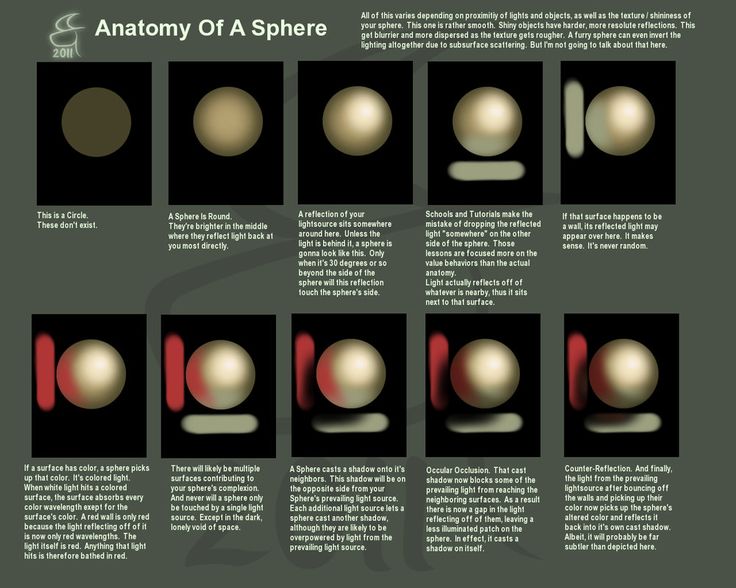
If light is not absorbed by a surface, it is mostly reflected. Reflection occurs when incoming solar radiation bounces back from an object or surface that it strikes in the atmosphere, on land, or water, and is not transformed into heat. The proportion of incoming solar radiation that is reflected by the Earth is known as its albedo. Overall, Earth reflects about 29% of the incoming solar radiation, and therefore, we say the Earth’s average albedo is 0.29.
Snow and ice, airborne particles, and certain gases have high albedos and reflect different amounts of sunlight back into space. Low, thick clouds are reflective and can block sunlight from reaching the Earth’s surface, while high, thin clouds can contribute to the greenhouse effect.
The proportion of sunlight that’s reflected vs. absorbed, the re-radiation of heat, and the intensity of the greenhouse effect influence the amount of energy in the Earth system and global processes such as the water cycle and atmospheric and ocean circulation.
This diagram shows the percentage of sunlight that is reflected by different Earth surfaces or clouds.
Earth system models about the absorption and reflection of sunlight
This Earth system model is one way to represent the essential processes and interactions related to the absorption and reflection of sunlight. Hover over the icons for brief explanations; click on the icons to learn more about each topic. Download the Earth system models on this page.
This model shows some of the changes to Earth’s surface and atmosphere that can affect the amount of sunlight that is absorbed or reflected. These changes influence the amount of heat that is re-radiated, and can also greatly influence the biosphere by altering the amount of sunlight available for photosynthesis.
How human activities influence the absorption and reflection of sunlight
The Earth system model below includes some of the ways that human activities directly affect the amount of sunlight that is absorbed and reflected by Earth’s surface.
The Earth system model below includes additional ways that human activities directly affect the amount of sunlight that is absorbed and reflected by Earth’s atmosphere. Hover over or click on the icons to learn more about these human causes of change and how they influence the absorption and reflection of sunlight.
The Earth system model below shows how human pollutants and waste affect the ozone layer and the amount of ultraviolet sunlight that is absorbed by Earth’s upper atmosphere (the stratosphere). Hover over or click on the icons to learn more about these human causes of change and how they influence the absorption and reflection of sunlight.
Explore the Earth System
Click the icons and bolded terms (e.g. re-radiation of heat, airborne particles, etc.) on this page to learn more about these process and phenomena. Alternatively, explore the Understanding Global Change Infographic and find new topics that are of interest and/or locally relevant to you.
To learn more about teaching the absorption and reflection of sunlight, visit the Teaching Resources page.
Links to Learn More
- Earth’s Energy Budget
- Measuring Earth’s Albedo via Satellite/ CERES
- A brief description of NASA’s “Clouds and the Earth’s Radiant Energy System” (CERES) satellite instruments
- Woods Hole Oceanographic Institution: How tiny plants help make clouds
- Earth’s Albedo and Global Warming
- NOAA Science on a Sphere, Aerosols: Black carbon and sulfate
- Ozone: What is it, and why do we care about it?
Reflection, Transmission, and Absorption
Reflection is the process by which electromagnetic radiation is returned either at the boundary between two media (surface reflection) or at the interior of a medium (volume reflection), whereas transmission is the passage of electromagnetic radiation through a medium.
Absorption is the transformation of radiant power to another type of energy, usually heat, by interaction with matter.
Fig. II.14 – a-c: Direct, mixed and diffuse reflection d-f: direct, mixed and diffuse transmission
Fig.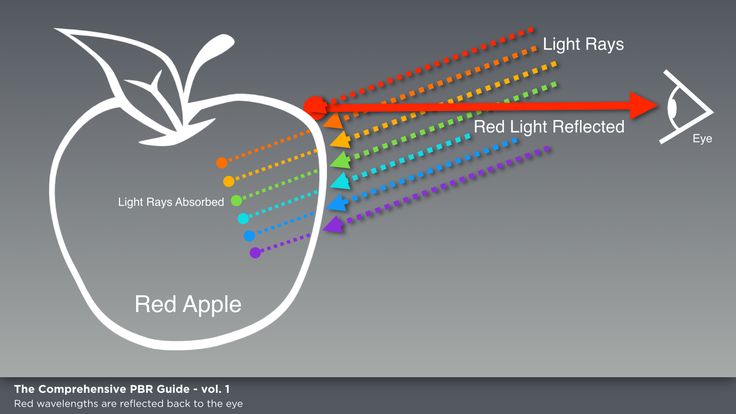
II.8.a. Reflectance r, Transmittance t, and Absorptance a
In general, reflection, transmission and absorption depend on the wavelength of the affected radiation. Thus, these three processes can either be quantified for monochromatic radiation (in this case, the adjective “spectral” is added to the respective quantity) or for a certain kind of polychromatic radiation. For the latter, the spectral distribution of the incident radiation has to be specified. In addition, reflectance, transmittance and absorptance might also depend on polarization and geometric distribution of the incident radiation, which therefore also have to be specified.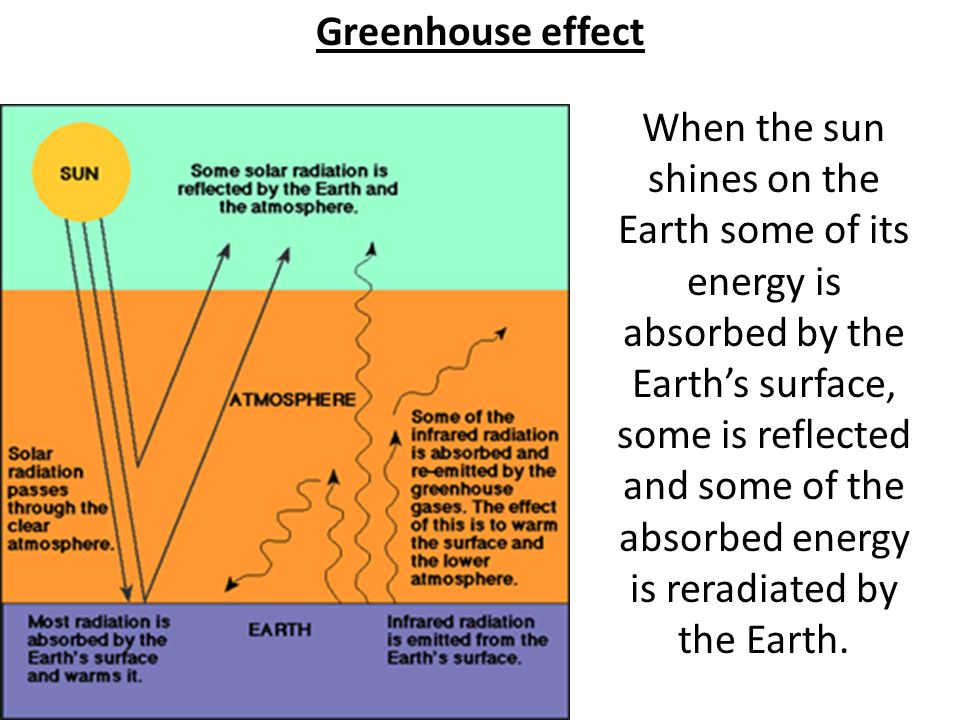
dFe,incident = Ee dA
and the (differential) reflected radiant power is given by the exitance Me, multiplied with the size of the surface element:
dFe,reflected = Me dA
Thus,
or
Me = r Ee
Total reflectance is further subdivided in regular reflectance rr and diffuse reflectance rd, which are given by the ratios of regularly (or specularly) reflected radiant power and diffusely reflected radiant power to incident radiant power. From this definition, it is obvious that
r = rr + rd
The transmittance t of a medium is defined by the ratio of transmitted radiant power to incident radiant power.
Again,
t = tr + td
The absorptance a of a medium is defined by the ratio of absorbed radiant power to incident radiant power.
Being defined as ratios of radiant power values, reflectance, transmittance and absorptance are dimensionless.
Quantities such as reflectance and transmittance are used to describe the optical properties of materials. The quantities can apply to either complex radiation or to monochromatic radiation.
The optical properties of materials are not a constant since they are dependent on many parameters such as:
• thickness of the sample
• surface conditions
• angle of incidence
• temperature
• the spectral composition of the radiation (CIE standard illuminants A, B, C, D65 and other illuminants D)
• polarization effects
The measurement of optical properties of materials using integrating spheres is described in DIN 5036-3 and CIE 130-1998.
Descriptions of the principle measurements are presented in paragraph III.1.f below.
II.8.b. Radiance coefficient qe, Bidirectional reflectance distribution function (BRDF)
The radiance coefficient qe characterizes the directional distribution of diffusely reflected radiation. In detail, the radiance coefficient depends on the direction of the reflected beam and is defined by the ratio of the radiance reflected in this direction to the total incident irradiance. In general, the reflected radiance is not independent from the directional distribution of the incident radiation, which thus has to be specified.
In the USA, the concept of Bidirectional reflectance distribution function BRDF is similar to the radiance coefficient. The only difference is that the BRDF is a function of the directions of the incident and the reflected beam (Fig. ). In detail, the (differential) irradiance dEe impinging from a certain direction causes the reflected radiance dLe in another direction, which is given by
dLe = BRDF · dEe
This BRDF depends on more arguments than the radiance coefficient.
The unit of radiance coefficient and BRDF is 1/steradian. The BRDF is often abbreviated by the Greek letter ρ, which bears the danger of mixing the BRDF up with reflectance (see foregoing paragraph).
Fig. II.16 – Geometry used for the definition of the bidirectional reflectance distribution function (BRDF). The BRDF depends on the directions of incident and reflected radiation, which are given by the angles Ji and Jr, which are measured relative to the reflecting surface’s normal, and the azimuth angles ji and jr, which are measured in the plane of the reflecting surface.
reflection and absorption of light – Blog – Ghenadie Sontu Fine Art
Light and color are the result of electromagnetic disturbances of some body, moving in space in waves.
Daylight consists of a wide variety of wavelengths. If you install a white screen in a dark room and pass a sunbeam through a small hole on it, then a bright spot of white color will appear on the screen. If, however, a glass prism is placed in the path of the sun’s ray, then a stripe painted in the colors of the rainbow will appear on the screen. It is called the solar spectrum, which is the result of the decomposition of sunlight into its component parts.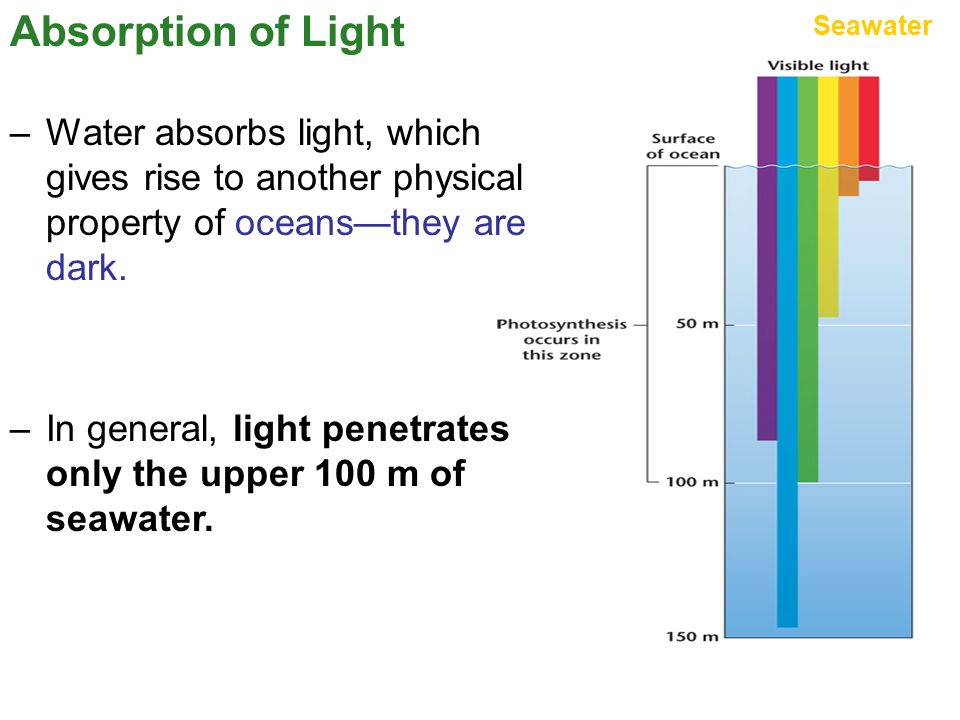
A beam of light passes through a prism and is split into a spectrum
The light wave of each color has its own length and frequency of oscillation. Light waves themselves have no color. Color comes from the perception of our eyes and brain.
The color of the surrounding material objects is formed from the absorption and reflection of light waves. White light hits an object, some of the light waves are absorbed, some are reflected. If all the waves are absorbed, then we will see black. If all the waves are reflected, we will see white light. If waves of a certain length and frequency are reflected, then we will see the color corresponding to this light wave:
All colors of the spectrum except orange are absorbed.
The origin of color from light rays is formed by two basic natures of color:
Additive colors – colors formed by outgoing light rays. Traffic lights, television, computers, telephones work on the basis of additive color rendering – wherever colored light rays and effects based on them are used.
Subtractive colors are colors formed by the reflection of light from material objects. In fact, this is everything material and material that surrounds us, both created by nature and created by man. This nature of color has another name – pigment color, and substances that affect and change color are called pigments.
In addition to the method of formation of color, the two color natures have significant differences – these are the primary colors and the method of formation of additional colors. Let’s take a closer look at how this happens.
The solar spectrum visible to the eye consists of seven primary colors: red, orange, yellow, green, blue, indigo and violet.
The spectrum known to us contains primary and secondary colors. And here comes the essential difference between additive and subtractive colors:
Primary additive colors – green, blue, red. If you combine additive colors, then a white color is formed, in the absence of color rays a black color is formed. Complementary colors are formed by combining the rays of the three primary. The example shows that when red and green additive colors are combined, yellow is formed, when red and blue are combined – purple, when the primary three colors are combined – white. :
An example of the RGB color model. The combination of green and red forms yellow.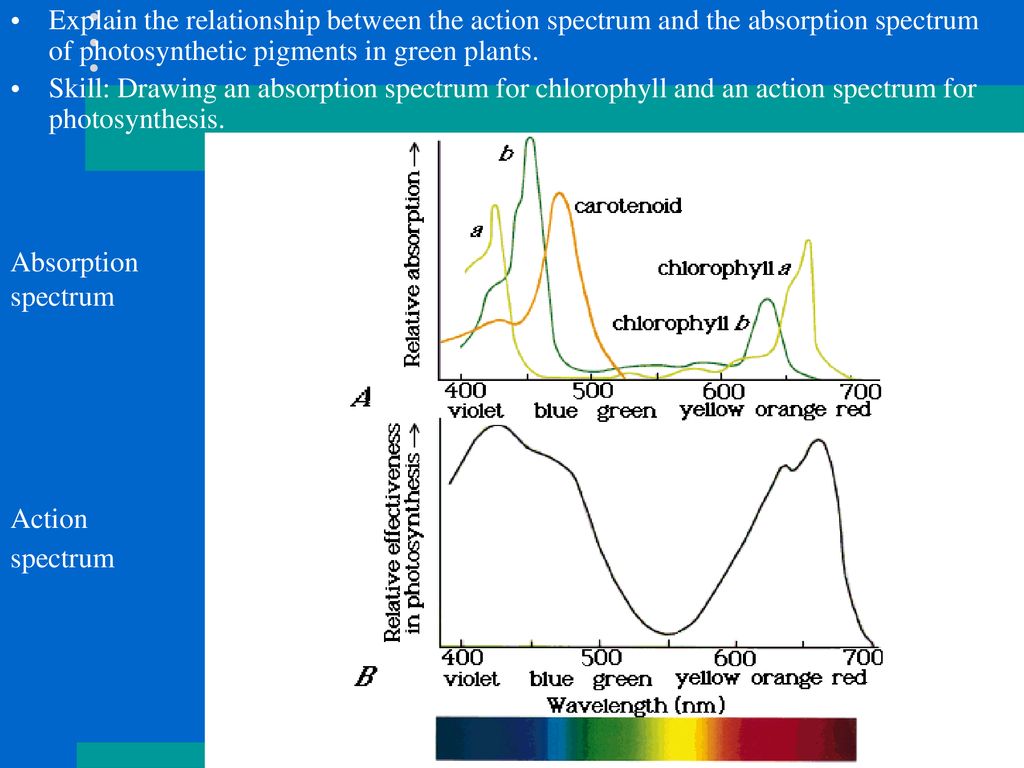
Basic subtractive colors – yellow, blue, red. If you mix the primary subtractive colors, then black is formed. Complementary colors are formed by mixing pigments of primary colors. For example, when red and yellow subtractive colors are combined, orange is formed, when red and blue are combined – purple, when the main three colors are combined – black:
The combination of three primary colors: blue, red, yellow form black. The combination of yellow and blue is green.
In the left half of the spectrum – from red to half green – are the so-called warm colors , and in the right half – from the middle of green to purple – cold colors. The warmth of colors increases from the middle of the spectrum towards red, their coldness towards violet. Green approaching yellow has a warm tint, and approaching blue a cold tint.
Chromatic colors are all colors of the spectrum and the color shades derived from them. In the example, the colors are arranged according to the way they are formed. The triangle inside is formed by the primary colors: red, yellow, blue. Further, three triangles of additional colors included in the spectrum formed by a combination of primary colors adjoin the triangle of primary colors: green – from blue and yellow, orange – from red and yellow, violet – from red and blue. The outer ring is formed from a combination of primary colors, secondary colors and intermediate colors formed from their mixing.
Achromatic colors – Colors that do not have a hue and differ from each other only in lightness (white, gray, black) are called achromatic; all other colors differ not only in lightness, but also in chromaticity, they are called chromatic.
Color has three main properties: lightness (luminosity), hue (color) and saturation (intensity, purity, brightness).
The lightness of the color is determined by the amount of reflected light. Achromatic colors – white, gray, black – have different lightness. The same applies to chromatic colors: if you compare them with each other, you will see that some of them are lighter, others are darker.
When a paint is mixed with white or black, it becomes a lighter or darker color of the same hue.
A strongly lightened violet color closer to lilac and has little resemblance to spectral violet.
If you mix paint with gray equal to it in lightness, you can get tones from purer to blacker, depending on the amount of gray entered. The degree of difference between a chromatic color and an achromatic color equal to it in lightness is called saturation.
We feel all the variety of colors because different bodies reflect only certain light rays that we perceive with our eyes in the form of rays of different colors. Therefore, light is a physical phenomenon, and the sensation of color that occurs when a visual color is irritated is a physiological phenomenon.
Transparent bodies do not block rays and let them pass through them, opaque bodies reflect them. Therefore, the different coloring of objects is due to the fact that they have a different ability to absorb and reflect certain rays of sunlight.
The impression of visual color occurs when an object reflects a green ray and absorbs all others. The black color is the result of the complete absorption of all rays. With partial absorption and partial reflection of rays by the body, it appears gray. If more rays are absorbed than reflected by the body, then its gray color is perceived as darker, in the opposite situation – lighter.
The sensation of white color occurs when all the rays of the spectrum are reflected or optically mixed.
Paints do not completely reflect the beam of any particular color and do not completely absorb all the others, therefore, in terms of purity and saturation of colors, they cannot be compared with the colors of the solar spectrum. Therefore, when mixing paints with paints, their additional colors of white cannot be obtained, and when mixing paints of all colors, a mixture of dirty, almost black color is formed.
When mixing colors of non-complementary colors, intermediate colors are obtained, for example, a mixture of red and yellow gives orange, yellow and green – yellow-green, blue and red – purple. In the latter case, the violet color, if the colors of the spectrum are arranged not in the form of a straight band, but in the form of a circle, will occupy a place in the spectrum between red and blue, which are in contact with each other.
The desired color can be obtained in two ways: by making mixtures of paints and by applying paints in transparent layers one on top of the other.
When applying paints in thin transparent layers on top of each other – when glazed – optical mixing of colors occurs . Its essence is as follows.
If a transparent layer of blue oil paint is applied on a white ground, and another layer of transparent yellow paint is applied on top, then the light falling on the upper transparent layer will hardly be reflected and will pass through it further, and some rays will be absorbed inside the yellow paint layer, and in contact with the underlying blue paint, the light will no longer be white, but yellow. Penetrating further through the layer of blue transparent paint, the yellow-colored light also undergoes absorption of some rays, as a result of which the light of yellow and blue, i.e. green, reaches the white opaque paint of the ground, is reflected from it and passes back through a layer of blue and then yellow paint.
During mechanical mixing of paints, light is partially reflected from the surface of the paint layer, partially refracted, passes into the depth of the layer, and as light penetrates into the paint mixture, the light rays are more and more absorbed by the particles of paint pigments, therefore, only very little light. Reflected from the paint layer are only those rays that are not absorbed by the mixed paints.
Therefore, when performing their creative work and solving color problems, artists use not only mixtures of colors obtained on the palette, but also resort to glazes, which give greater expressiveness, purity, depth and lightness of color tones.
The human eye perceives the strength and color composition of light not only naturally, unconsciously, as they enter it from outside. The eye has the ability to adapt to the light and color acting on it, not only as a whole, but also by separate parts of its retina. This ability of the eye explains the phenomenon of 90,008 color contrasts. If you look closely at a painted surface, and then look at another painted surface with a different light intensity, then this will affect the power of perception of the color of the second surface by the eye, namely: if the first surface is darker than the second, then this latter will appear lighter than she really is. After focusing the gaze on any color in the eye, the impression of a so-called sequential contrasting color, very close to the complementary color, arises.
Reflections are the result of an object reflecting the color of another object painted in a different color, which changes the natural color of the first and gives it its own shade. The reflex can be more or less significant, depending on the strength of the illumination, the brightness of the color, its surface, and the proximity or distance of objects from each other.
The correct transmission of reflexes plays a very important role in painting, especially if the work is done en plein air.
In Education through Art, painting technology, Art School, Art School, watercolor school
Tags The nature of color and light, Light and color, Primary colors, Warm and cold colors, Achromatic and chromatic colors, Lightness of color, Saturation, light reflection and absorption, Complementary colors, Optical color mixing, Color contrast, Reflexes, primary colors, What colors are called warm?, What is meant by color saturation?, What colors are called complementary?, Additive colors, Subtractive colors, complementary colors, Primary subtractive colors, colors – yellow, blue, red, Primary colors yellow, Colors and shades, Chromatic and achromatic colors , Color saturation, Reflection and absorption of color, color reproduction in painting
Color absorption.
Color absorption
The colors that we attribute to objects are the result of the radiation reflected by them, reaching our eyes. When illuminated with white light, a red brick appears red because it reflects radiation from the red part of the spectrum. It can reflect a lot of yellow and orange, some green, some purple and even blue. But most of the blue, violet and green radiation will be absorbed. You can accurately measure the color (spectral) reflection and absorption of any surface. Any color has its own spectral composition, whether it is an artificial dye or a natural color. Two colors that look almost the same to the eye may well have completely different spectral compositions.
The standard Kodak test chart allows the photographer to control the reproduction of bright and pastel colors, as well as the contrast and effect of color filters.
Pure (bright) colors are usually the result of selective (highly selective) absorption and reflection.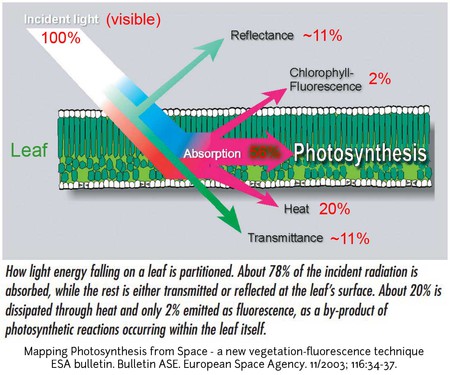
Dimmed colors are the result of generally low reflectivity, where nearly all wavelengths are absorbed and only a few are reflected. Such colors can be considered as some kind of pure colors mixed with black. From a photographic point of view, neither a muted nor a pastel color can be turned into a bright or saturated color. A color heavily saturated with white light can be dimmed, then it will turn into a muted gloomy shadow. A color with an excess of neutral density (an admixture of “gray”) can be made lighter, but at the same time it becomes a faded shadow.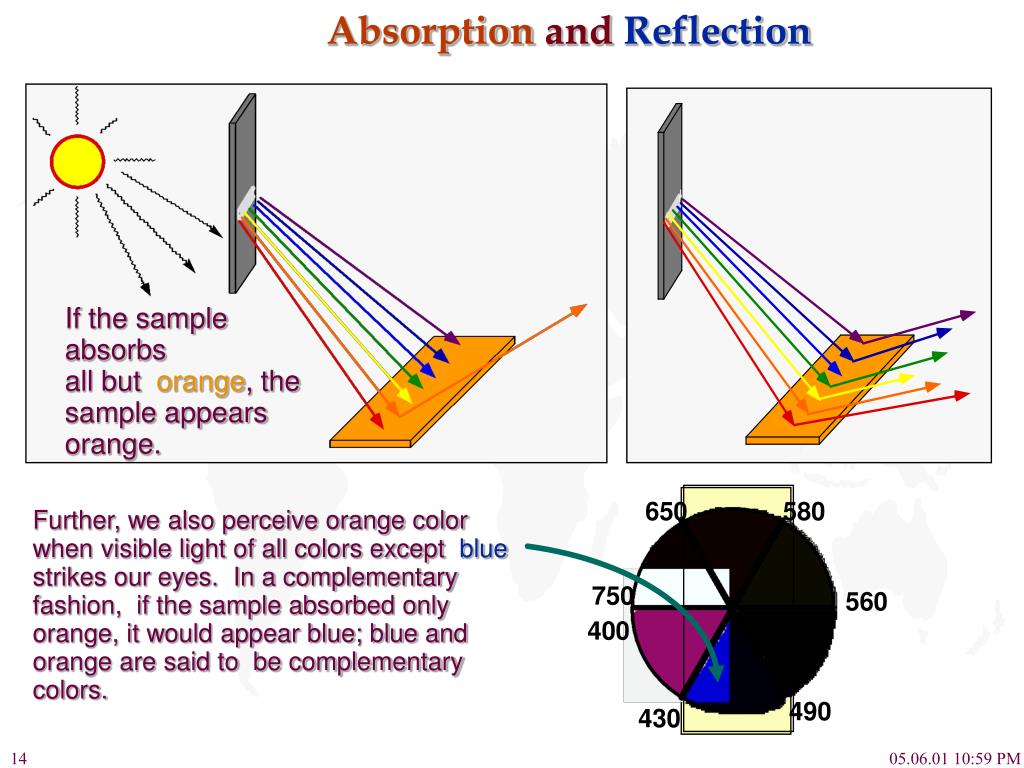
Relative illumination also has a strong influence. In the shade, the color looks less bright than the same color next to it in full sunlight. In the photograph for both cases separately, you can achieve the same color saturation by individual selection of exposure. If you shoot a plot that has both lights and deep shadows at the same time, then when transferring color, you will have to give preference to one of the options – either lights or shadows. The reason that many colored surfaces look less vibrant on overcast days is surface reflection, not light levels. A cloudy sky is reflected, and a completely diffused light gives a completely diffused sheen. Direct sunlight does not cause glare over a wide range of incidence angles and does not form a dazzling bright spot when looking at the surface “against the light.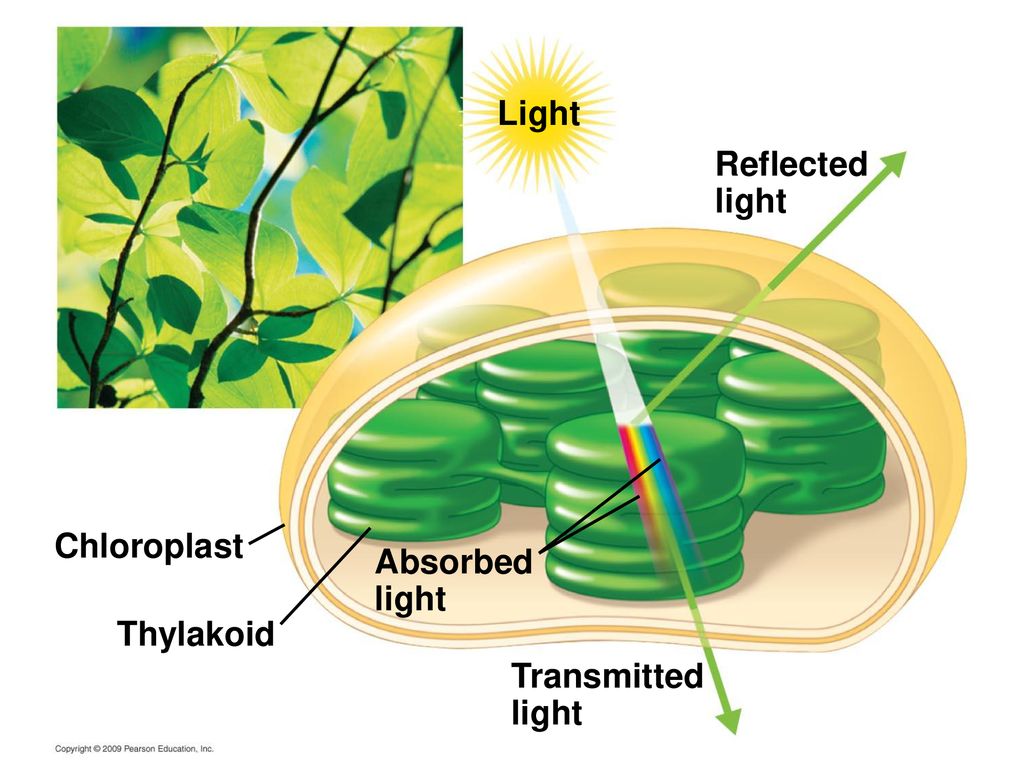
V. Color movement
V. Color movement
The sliding of our gaze over a color-covered palette leads to two main results: 1) a purely physical effect of color occurs when the eye is fascinated by its beauty and its other properties. The viewer experiences a sense of satisfaction,
Chapter 2 The Art of Color
Chapter 2 The Art of Color
The perception of an image is largely determined by color. Color makes the image more expressive, conveys the mood, sharpens perception, gives the form a special significance and spirituality. A person is constantly influenced by color
2.1. The Meaning of Color in Fine Art
2.1. The meaning of color in the visual arts
The world is beautiful because a person perceives it in color.
2.2. Nature color
2.2. The nature of color
Color is a very complex phenomenon. There are several completely different approaches to its study. Physicists investigate the energy of electromagnetic oscillations, measure the length of a color wave, analyze the spectrum. Chemists work with dyes, study them
2.8. Emotional impact of color
2.8. The emotional impact of color
Color perception is subjective. A color characteristic can be given to any natural phenomenon, smell, taste, sound. The more developed the artist’s “sensitivity” to color, the more accurately any color compositions will be performed by him.
8. M&A
8.
If you think about it, we are endlessly busy waiting for others.
Ilya Lagutenko
A few days after the presentation of the Meamurs, the Trolls left for Kyiv to perform at the Just Rock festival. It so happened that Ilya and the musicians were traveling in the same
Color image
color image
With the advent and development of color photography, the masters of Soviet photography faced the problem of color organization, color solution, coloring of a photographic image. The concept of “color” came to photography from painting, where this term
MATISSE. COLOR MASTER
MATISS. MASTER OF COLOR
The pictorial color revolution lasted more than half a century. Matisse said goodbye to life at 1954 He gave a lot to people; his light, cheerful art testifies that the avant-garde was diverse, could not only frighten or lead into labyrinths
Color and volume axis*
Axis of color and volume*
Starting the organization and reorganization of the general artistic construction machine in the State, attention was paid to the creation of a network of museums as centers of propaganda and education of the broad masses.
What color is the cast iron?
What color is cast iron?
Try it, guess! Ural children usually answer – black. Do you think so too, remembering your grandmother’s favorite frying pan, tucked into the kitchen among smart Teflon girlfriends? No, my friend, this black frying pan is not by itself. She’s just
ABSORPTION OF LIGHT • Big Russian Encyclopedia
Authors: А. The electromagnetic field of the light wave excites additional. vibrations of electrons and ions of a substance, for which energy is spent. Partially it returns in the form of secondary electromagnetic radiation. In terms of quantum theory, the process of P. s. associated with the transition of electrons in atoms and molecules that absorb radiation from low energy levels to higher ones. The reverse transition to the ground or lower excited state can be performed with the emission of a photon, or non-radiatively, or in a combined way, and the method of the reverse transition determines into what form of energy the energy of the absorbed light passes.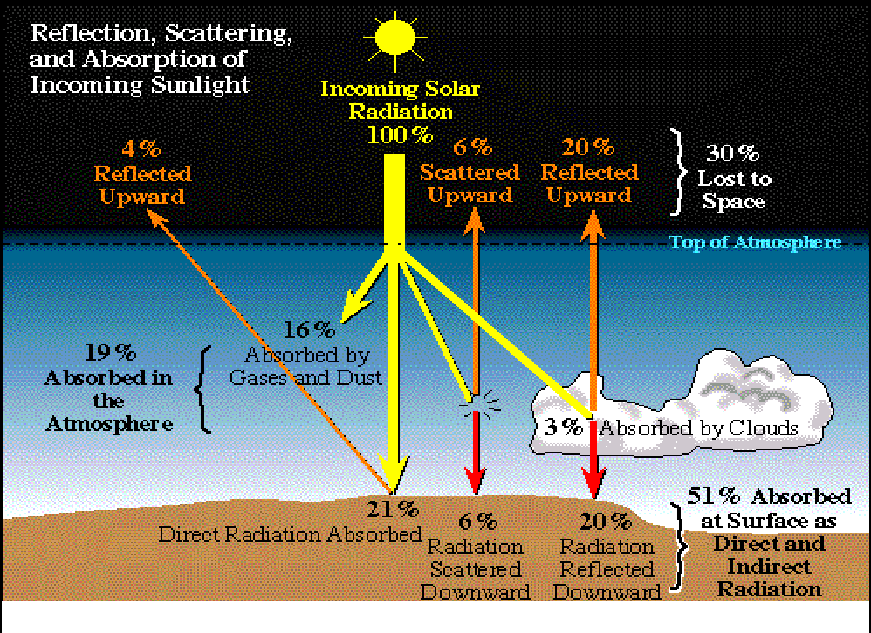
Usually, the light intensity I decreases with increasing distance l traveled in the substance according to the exponential law: I=I 0 e –α l , where I 0 is the initial light intensity, α is the absorption index, depending on transparency of the environment. This law was experimentally established by P. Bouguer in 1729 and theoretically derived by I. Lambert in 1760 (see Bouguer – Lambert – Beer law).
The dependence of the absorption index α on the wavelength of light λ is called the absorption spectrum of a substance. The absorption spectrum of isolated atoms (for example, atoms of rarefied gases) consists of narrow spectral lines, i.e., the absorption coefficient α is nonzero only in certain narrow wavelength ranges (0.1–1 nm wide) corresponding to eigenfrequencies. vibrations of electrons inside atoms. The molecular absorption spectrum, determined by the vibrations of atoms in molecules, consists of absorption bands (10 nm – 10 µm wide).
In conducting media (metals, plasma) interaction with light means. degree is determined by free electrons, so α depends on the electrical conductivity of the medium. P. s. in conducting media, it strongly affects all processes of light propagation in them; formally, this is taken into account by the fact that the term containing α enters the expression for the complex refractive index of the medium. The incident light wave is absorbed almost completely in a thin (about 10 nm) layer; its energy is converted into the energy of motion of the electron plasma.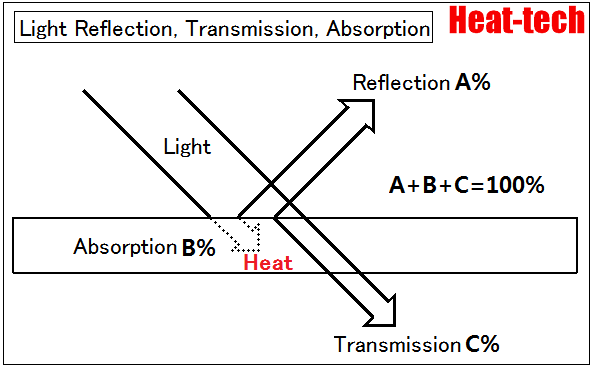
When light is absorbed by molecules of a substance dissolved in a practically non-absorbing solvent, or by gas molecules, the exponent α turns out to be proportional to the number of absorbing molecules per unit length of the path of the light wave, or, which is the same, per unit volume filled with transmitted light, i.e. is proportional to the concentration C of the dissolved substance (established by the German scientist A. Behr, 1852). In real gases and solutions, this is not always the case.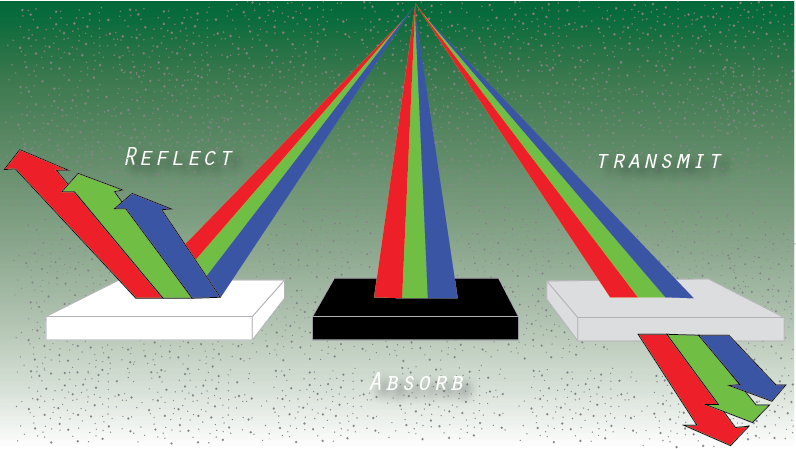
Absorption spectra can be so individual that they can effectively control the chemical. composition of solutions. For example, an adequate way to verify the authenticity of alcoholic products is to measure absorption spectra, individual not only for producers, but also for the soil on which the grapes grew.
At high light intensities, nonlinear effects begin to appear, α becomes a function of the light intensity, and Bouguer’s law is violated (nonlinear PS). Such effects can also occur when several photons are simultaneously absorbed (see Ref. Multiphoton absorption of light ). In this case, the intensity of the fluctuating photon flux stabilizes; The extraction of photons from the original beam can occur in pairs (two-photon absorption), triplets, etc. The beam thinning occurs in places of the highest photon concentration, i.e., in fluctuation bursts of intensity. As a result, the bursts are smoothed out and the photon flux becomes more regular. In such nonlinear processes, the efficiency of photon fluctuation suppression is low; nevertheless, they make it possible to reduce photon noise even below the noise level of an ideal laser, which is especially important in high-precision optical systems.
The opposite process is the nonlinear saturation effect, due to the fact that a very large proportion of absorbing particles, having passed into an excited state and remaining in it for a relatively long time, loses the ability to absorb light, which noticeably changes the nature of the PS. environment. When almost all the electrons of a substance under the action of light pass into an excited state and there are no absorbing particles, the so-called enlightenment of the medium – the almost complete absence of absorption (see. Enlightenment effect).
If a population inversion is artificially created in an absorbing medium, then each photon from the incident flux has a greater probability of inducing the emission of exactly the same photon than of being absorbed by itself (see Stimulated Emission ). In this case, the intensity of the outgoing light exceeds the intensity of the incident light, i.e., light amplification takes place, and the absorption index α becomes negative, so this phenomenon is called negative P.